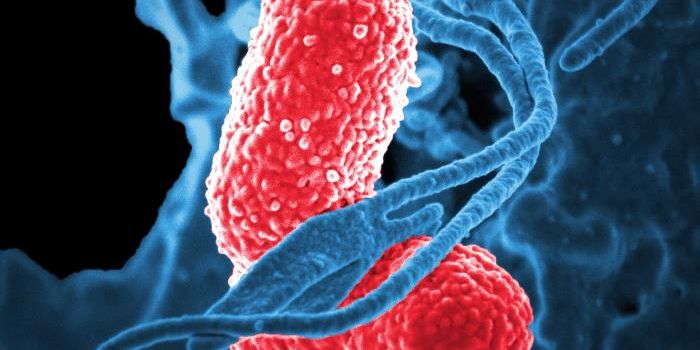
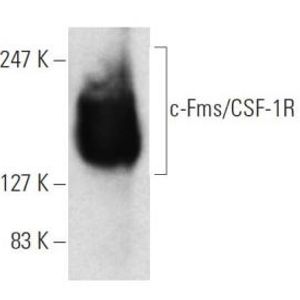
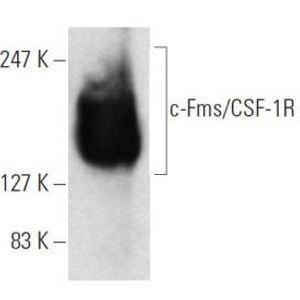
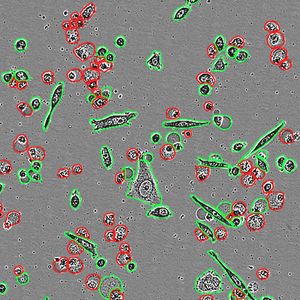
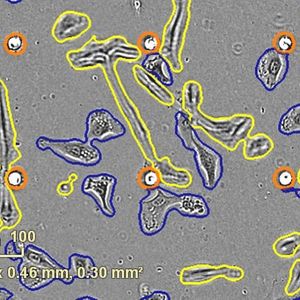
By modeling microsporidia infection in roundworms, scientists have determined that the single-celled fungi replicate by fusing their hosts’ cells together.
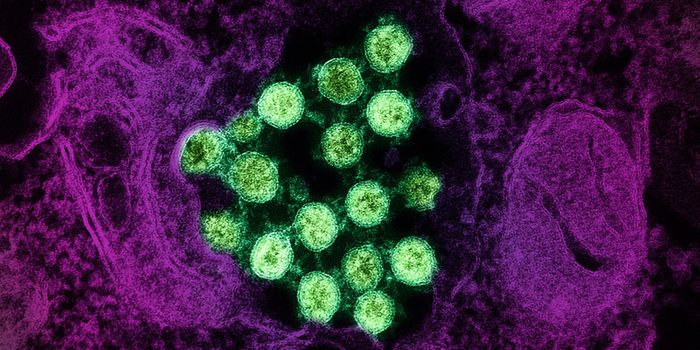
Microsporidia are single celled parasitic microbes that infect both invertebrates and vertebrates. Microsporidia can invade human hosts and cause illnesses like microsporidiosis, primarily observed in people with compromised immune systems. It has a variable clinical presentation based on the species causing the infection; it most often features diarrhea. It can also be fatal. More research in this area is needed for a complete understanding of the disease.
Researchers at UC San Diego have
reported some advances in Nature Microbiology. They have found that microsporidia are able to join host cells together and in doing so, are allowed to multiply for rapid diffusion throughout uninfected cells.
"Viruses and bacteria have been shown to fuse host cells together to facilitate the spread of an infection," said Emily Troemel, the study leader and Professor of Biology at UC San Diego. "But this is the first time we've seen this mode of infection by a eukaryotic pathogen, which is a single-celled organism with a distinct nucleus containing genetic material."
"It's like microsporidia have figured out that, like soldiers fighting in an urban warfare, it's easier and safer to go from house to house by entering adjacent houses through a common wall, rather than by going through the front door of each house," continued Troemel.
The typical home of microsporidia is in the gastrointestinal tract of animals, which makes studying the reproductive mechanism a bit challenging. Many other cells and microbes share that same space.
“We often don't know where pathogens invade or how they spread from cell to cell," commented Keir Balla, the first author of the study and a graduate student in Troemel's laboratory. "This is partly due to the complex arrangement of cells in the body. Because the gut is made up of millions of cells, it's virtually impossible to tell which gut cell was first invaded or the extent to which infection was able to spread. And this can prevent effective treatment."
The investigators utilized C. elegans - roundworms - a common model in biology. This gave them a simple and visually accessible way to study microsporidia in the gut. If you want to know more about the model, check out the video below.
"The entire body of this worm is roughly 10 times shorter than a single human eyelash and its gut is made up of just 20 cells," explained Balla. "But despite the drastic differences in the number of gut cells between the worms and humans, their gut structure is quite similar. This means there are probably parallels in how microsporidian infections spread in both the human and worm cells."
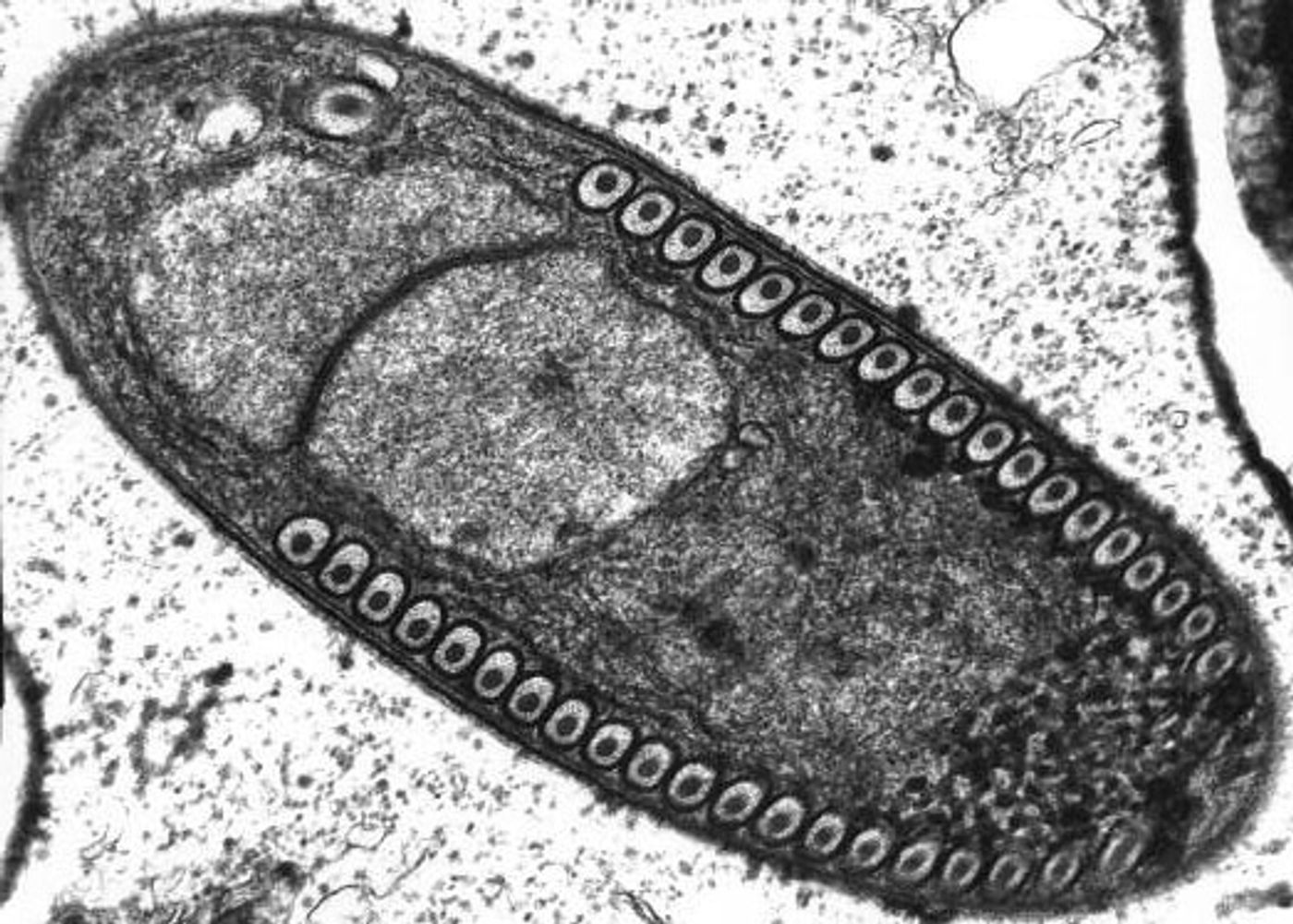
Using fluorescent labels to differentially tag the RNA of microsporidia and cell membranes, the scientists tracked a single infection from one microsporidia microbe in a single gut cell. It multiplied into over 50,000 progeny and spread throughout all 20 cells of the worm gut, demonstrating how infection with only one cell can take over the entire intestinal tract.
"While watching these infections in the living worms, we saw the microsporidia grow and spread beyond the initially invaded cell by fusing neighboring gut cells together," said Troemel. "This process continued across all of the gut cells until the majority of the entire intestine was fused into one giant cell filled with microsporidia."
There are currently no effective treatments for this infection, though it is widespread. This research may help change that.
"Our observation in this study that a single microbe can fuse many host cells into one should provide insights for other scientists designing more effective treatments for microsporidia infections. Because these pathogens remain hidden within their hosts' cells, treatments will need to be designed that deliver drugs or other agents to these intracellular regions," concluded Balla.
Sources:
AAAS/Eurekalert! via
UC San Diego,
Stanford University,
CDC,
Nature Microbiology