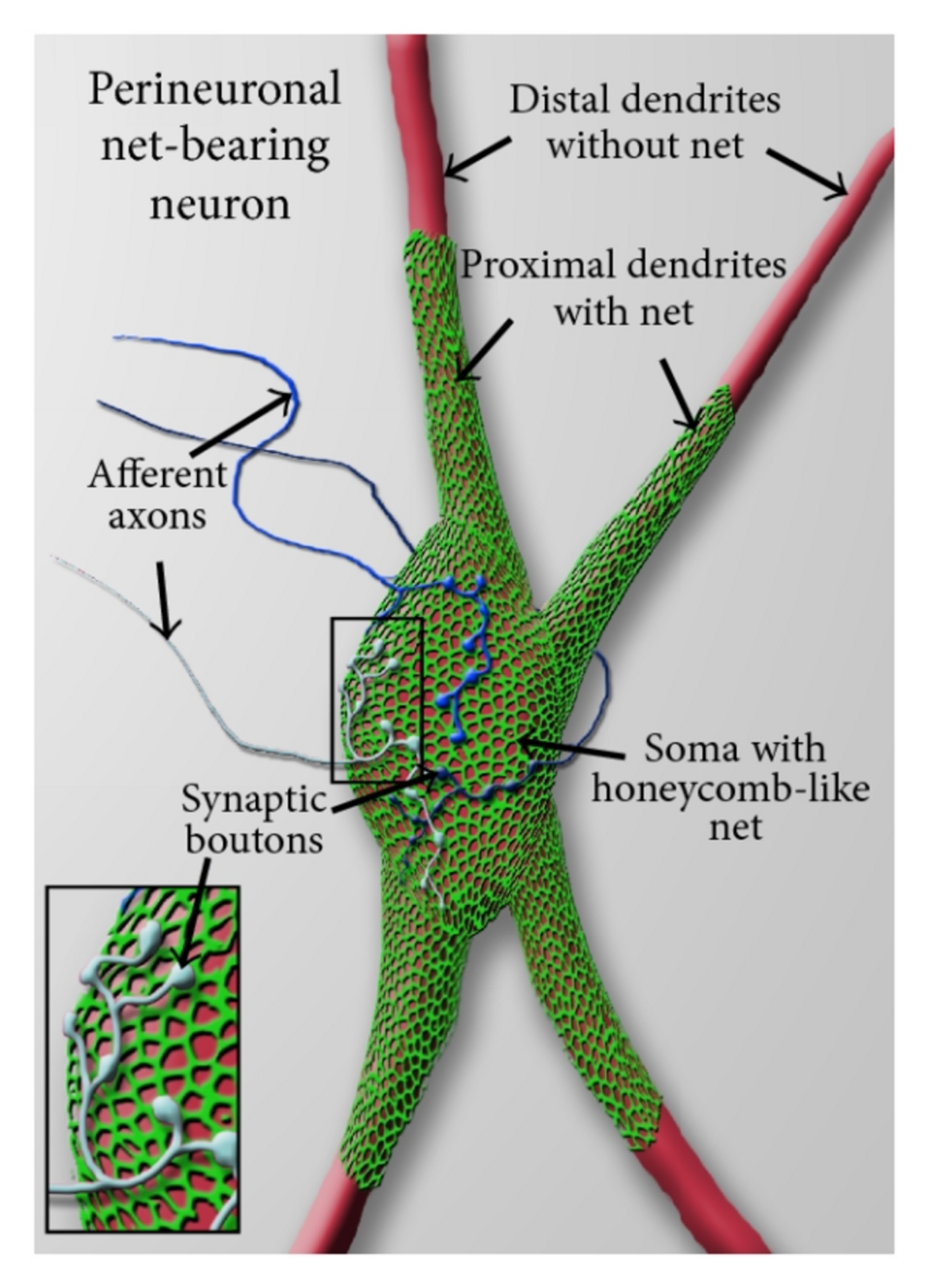
Perineuronal nets (PNNs), a specialized extracellular matrix structures that surround the cell bodies and proximal dendrite of a subgroup of central nervous system neurons. Although known since 1893, described by Golgi, the studies to understand the structure did not pick up until the last decade. PNNs were found to play a critical in neuroprotection, neuroplasticity, epilepsy, stroke, and Alzheimer’s diseases. However, the mechanism of action remained elusive until now.
Schematic view of a perineuronal net-bearing neuron. Credit: de Winter et al., Neural Plasticity. 2016
A research team led by Harald Sontheimer, the director of the Virginia Tech Carilion Research Institute Center for Glial Biology in Health, Disease, and Cancer and the executive director of the School of Neuroscience, part of the Virginia Tech College of Science, has determined the nets modulate electrical impulses in the brain. What’s more, brain seizures can occur if the nets are dissolved. The findings are reported in Nature Communications,
“We started by investigating tumor-associated epilepsy, and we accidentally learned something else important about how the brain works in disease and health,” Sontheimer said.
The group studied brain tumor, glioma-associated epileptic seizures and associated the reduction in peritumoral fast-spiking interneuronal (FSNs) activity to degradation of PNNs that surround them.
“Unexpectedly, we also saw the enzyme attacking the perineuronal nets,” Sontheimer said, noting that the tumor released proteolytic enzymes degrade PNNs. “It was a surprise to see this bystander effect of seizure activity once the neurons were stripped of their nets.” To further delineate the effect of the tumor from the tumor released enzyme associated PNNs disruption, the researchers introduced the enzyme in naïve healthy animal brains. The enzymatic degradation of PNNs resulted in seizures, confirming that despite no loss of neurons was seen in non-tumor animals, seizures were induced.
“Without the perineuronal nets, inhibitory neurons would fire too slowly, and therefore inhibition becomes too little, too late, and a seizure will occur – even in otherwise healthy brains,” Sontheimer said, noting that the enzyme can devour a perineuronal net in less than 30 minutes. “No one thought that these structures would have such a profound effect on how normal processes operate.”
The authors suppose that PNNs are akin to myelin sheaths around axons, in that their disruption reduces specific membrane capacitance and results in FSNs abnormal firing rates.
“Importantly, the finding that tumor-induced disruption in perineuronal nets contributes to imbalanced inhibitory neurotransmission suggests a new target for therapeutic intervention to control tumor-associated seizures,” said H. Steve White, a professor, and chair of the Department of Pharmacy in the University of Washington’s School of Pharmacy in Seattle, Washington.
White, a renowned expert in epilepsy and anticonvulsant drug development research, was not involved in Sontheimer’s study.
“Although additional studies are needed, it is likely that the findings reported by Dr. Sontheimer and his team are applicable to other forms of acquired epilepsy where brain injury is associated with a heightened inflammatory response,” White said, noting that the implications for treatment and prevention of epilepsy are particularly striking since current therapies are aimed at controlling seizures. “While controlling the symptoms of the disease is significant, the results of this study suggest a possible path toward modifying the development and progression of epilepsy, which would lessen the overall burden to the patient.”
More than 50 million people worldwide have epilepsy, according to the World Health Organization. About a third of those individuals don’t respond to current anti-epileptic treatments.
“If we confirm our hypothesis that digested perineuronal nets are responsible for other forms of acquired epilepsy, then a potential treatment could be an enzyme inhibitor,” Sontheimer said.
He noted that one such an inhibitor is already approved by the FDA for other uses, but he also cautioned that there’s a significant amount of research to conduct before their hypothesis is confirmed.
“We need new approaches to treat epilepsy. I think this could be an effective way to control seizures,” Sontheimer said. “And we solved a 125-year-old neuroscience mystery! This is what basic science is all about — keeping an open and observant mind to answer questions old and new.”
Sources: Virginia Tech News, Nature Communications, Science Daily
-
MAY 07, 2024Is It Anti-RNP or Anti-Sm/RNP?
- See More
-
APR 30, 2024Immuno-Oncology Virtual Event Series 2024
-
MAY 07, 20243rd International Biosecurity Virtual Symposium
-
JUN 06, 2024The Future of Scientific Conferencing
- See More