No More Antibodies: COVID Test Uses Glowing Nanotubes
Forget expensive antibody-based COVID diagnostics and long waits for test results. An innovative new approach developed by scientists and engineers at MIT leverages the power of nanotechnology to detect traces of the coronavirus in patient samples. The results are ready in around five minutes.
The emergence of these and similar technologies herald the next wave in biorecognition platforms, opening up exciting possibilities in the realms of health technologies and therapeutics.
As the pandemic drags on, vaccine rollouts continue across many parts of the globe. However, experts predict that diagnostic technologies will continue to be a cornerstone of public health responses to the COVID-19 crisis. For example, with travel, large social events, and workplaces opening up again, we will need cost-effective screening tools to keep our communities safe.
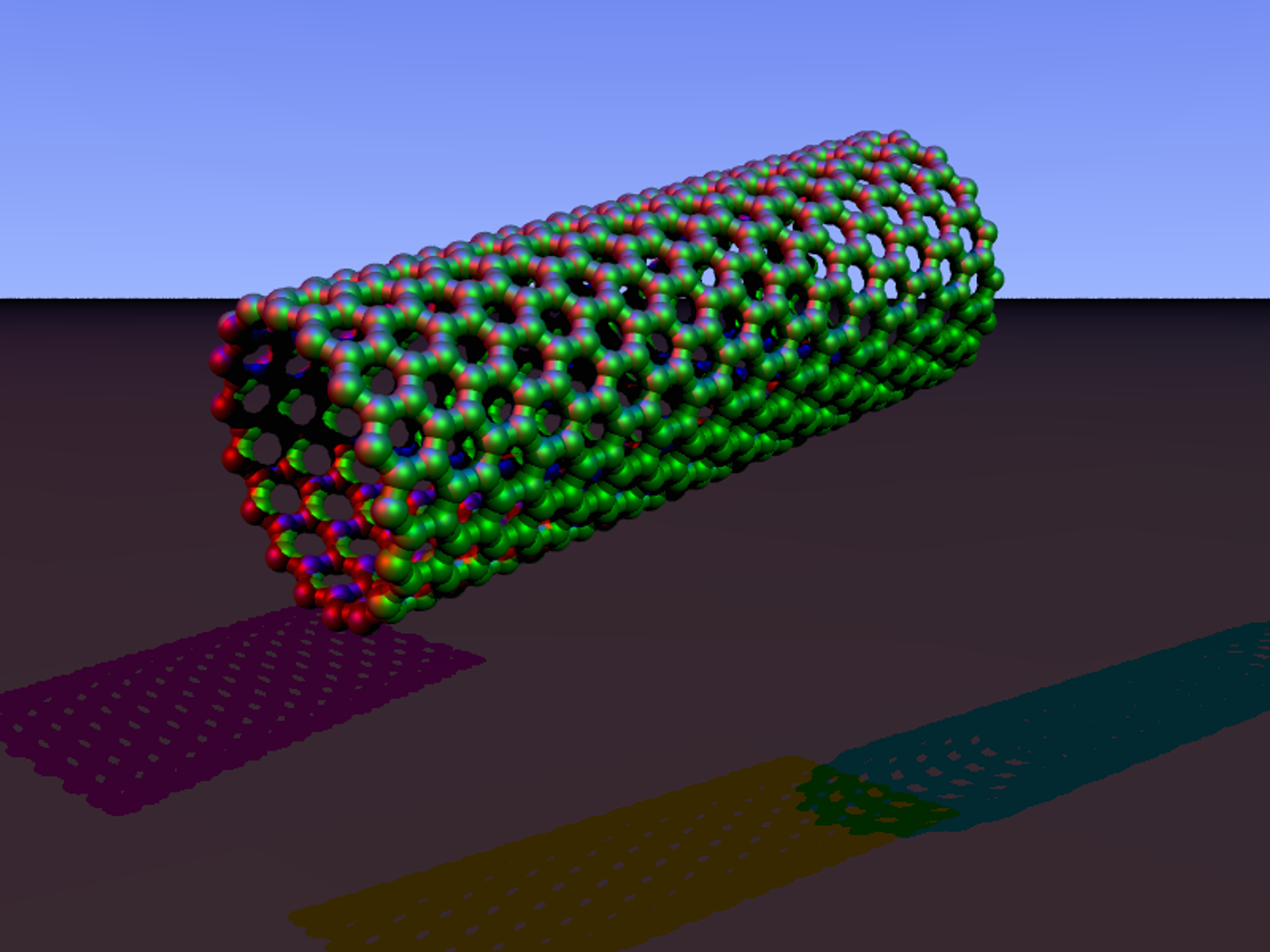
With that objective in mind, the team turned to a nanotechnology method of creating carbon nanotubes cocooned in polymer fibers. The target molecules (in this case SARS-CoV-2 proteins) stick to this network of fibers and alter the fluorescent readout generated when a laser shines on the nanotubes.
This sensor setup is known as Corona Phase Molecular Recognition or CoPhMoRe. It boasts speed, precision, and compatibility with saliva samples without the cost and development lag times of traditional antibody-based tests.
The nanotechnology powering this test had been in development long before the pandemic began. However, the innovators designed it such that it can be rapidly adapted to detect a novel viral target; their COVID test took under two weeks to set up.
According to the researchers, this is a significant step-up from first-generation COVID tests and has the potential to become the next gold standard in SARS-CoV-2 diagnostics. “It is a unique feature of this type of molecular recognition scheme that rapid design and testing is possible, unhindered by the development time and supply chain requirements of a conventional antibody or enzymatic receptor,” said a researcher involved in the study, Sooyeon Cho.
-
MAY 07, 2024Is It Anti-RNP or Anti-Sm/RNP?
- See More
-
APR 30, 2024Immuno-Oncology Virtual Event Series 2024
-
MAY 07, 20243rd International Biosecurity Virtual Symposium
-
JUN 06, 2024The Future of Scientific Conferencing
- See More