Iron is important to bacteria. Really important. It’s so important that bacteria secrete special molecules called siderophores to find iron and bring it back to the cell. It goes without saying, then, that iron is also important when bacteria infect a host.
Researchers studying Klebsiella pneumoniae at the University of Michigan at Ann Arbor knew that siderophores impact where and how Klebsiella infects its mammalian host. What they didn’t know was what specific effects those siderophores have on us mammals.
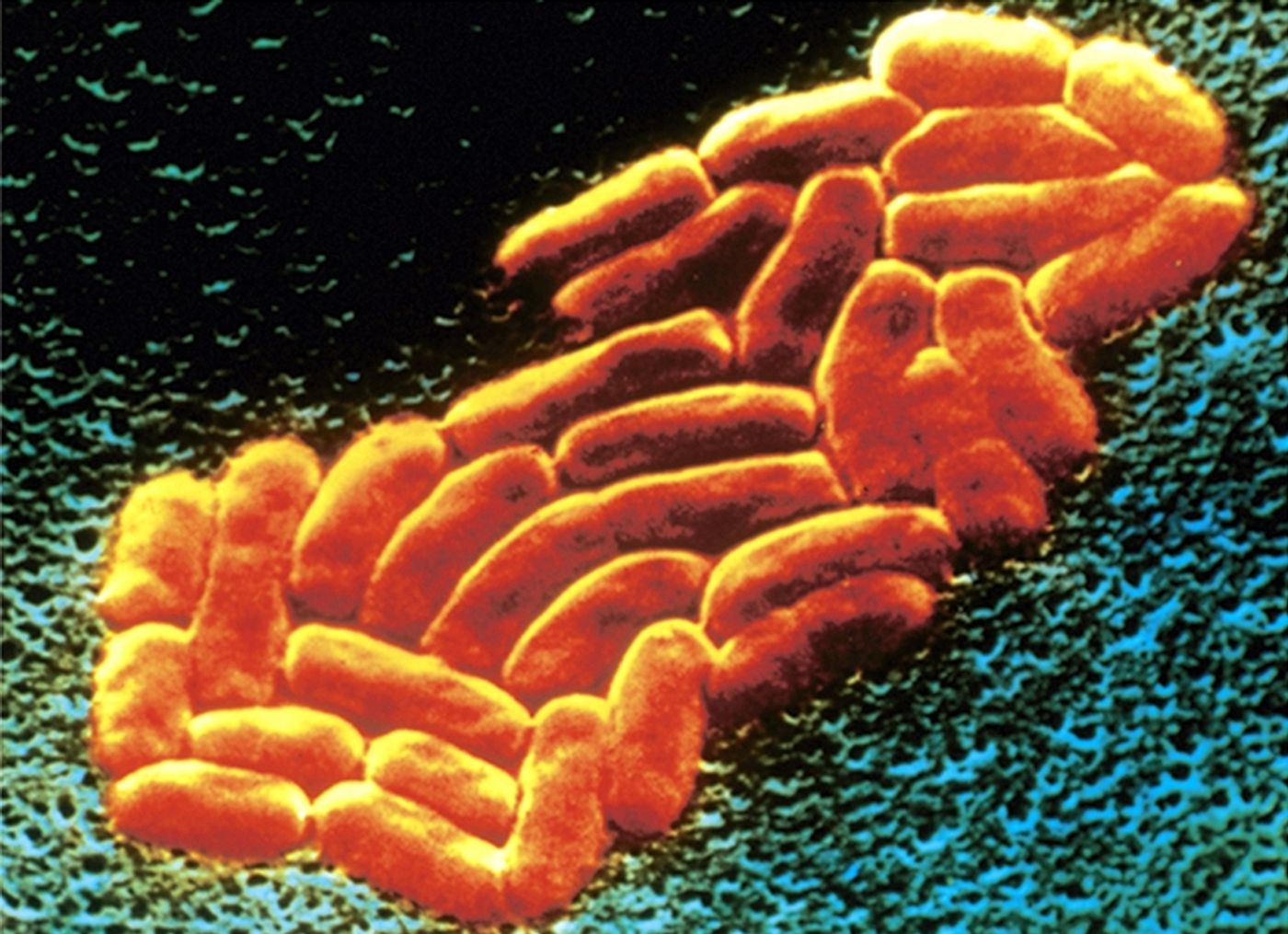
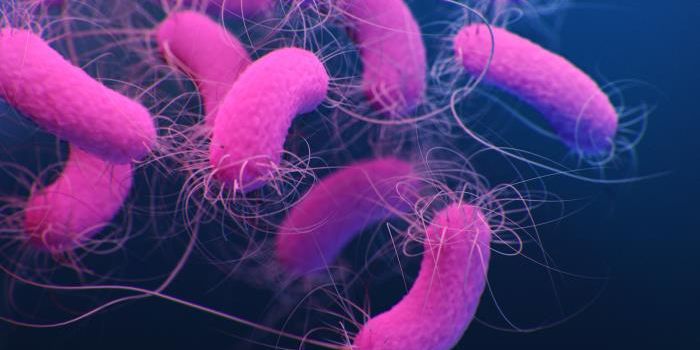
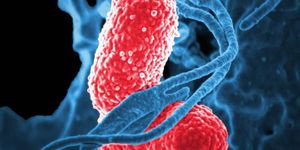
K. pneumoniae is a Gram-negative, rod-shaped bacterium. It’s one of the most common causes of hospital-acquired infections - these include pneumonia, urinary tract infections, and bacteremia. Once in the bloodstream, the mortality rate can be as high as 50% (often a result of antibiotic resistance).
The group, led by Michael A. Bachman, found that Klebsiella’s siderophores alter cytokine and chemokine secretion, stabilize the host factor HIF-1α, and help the bacteria disseminate to the spleen. According to Bachman, “we've known for a long time that siderophores are critical for bacteria to cause infection, because they steal iron from the host to grow. This study sheds some light on the consequences of that. When the bacterium steals this iron, what's likely happening is the host cells are becoming stressed and are inducing inflammation and cell signaling pathways that actually worsen the infection by allowing the bacteria to escape from the lungs to the spleen.”
In their initial experiments, they infected mice with wild type K. pneumoniae or a mutant that did not produce siderophores. They found that only bacteria that could make siderophores increased the expression of inflammatory cytokines and chemokines (IL-6, CXCL1, CXCL2, IL-1β, and MIP-3α) in the mouse lung.
Next, they wanted to know whether K. pneumoniae’s siderophores affected the stability of the transcription factor HIF-1α; this transcription factor regulates many host cell processes, including inflammation. The researchers engineered a fluorescent version of HIF-1α in order to monitor its stability in the mouse cells. Fluorescent HIF-1α was only produced when mice were infected with wild type K. pneumoniae, not when they were infected with bacteria that could not make siderophores. Interestingly, all three of K. pneumoniae’s siderophores had to be present for HIF-1α to remain stable. They also noticed that more bacteria disseminated from the lungs to the spleen when all three siderophores were present.
Based on this, the group wanted to know whether the bacteria needed to stabilize HIF-1α in order to disseminate effectively from the lungs to the spleen. To do this, they used transgenic mice in which HIF-1α expression in lung epithelial cells could be induced. Interestingly, the presence or absence of HIF-1α had no effect on bacterial growth in the lungs, but did affect dissemination to the spleen - there were roughly half as many bacteria in the spleen if HIF-1α was not expressed in the lungs.
Pretty interesting findings. According to Bachman, “these results indicate that bacterial siderophores directly alter the host response to pneumonia in addition to providing iron for bacterial growth. Therapies that disrupt production of siderophores could provide a two-pronged attack against
K. pneumoniae infection by preventing bacterial growth and preventing bacterial dissemination to the blood”.
Source: mBio,
EurekAlert