A big issue in developing treatments for cancer is how to target the cytotoxic drugs to the tumor. Thankfully, a group from the Department of Nanomedicine at Houston Methodist Research Institute have rationally designed a drug delivery system that effectively targets metastatic triple-negative breast cancer tumors.
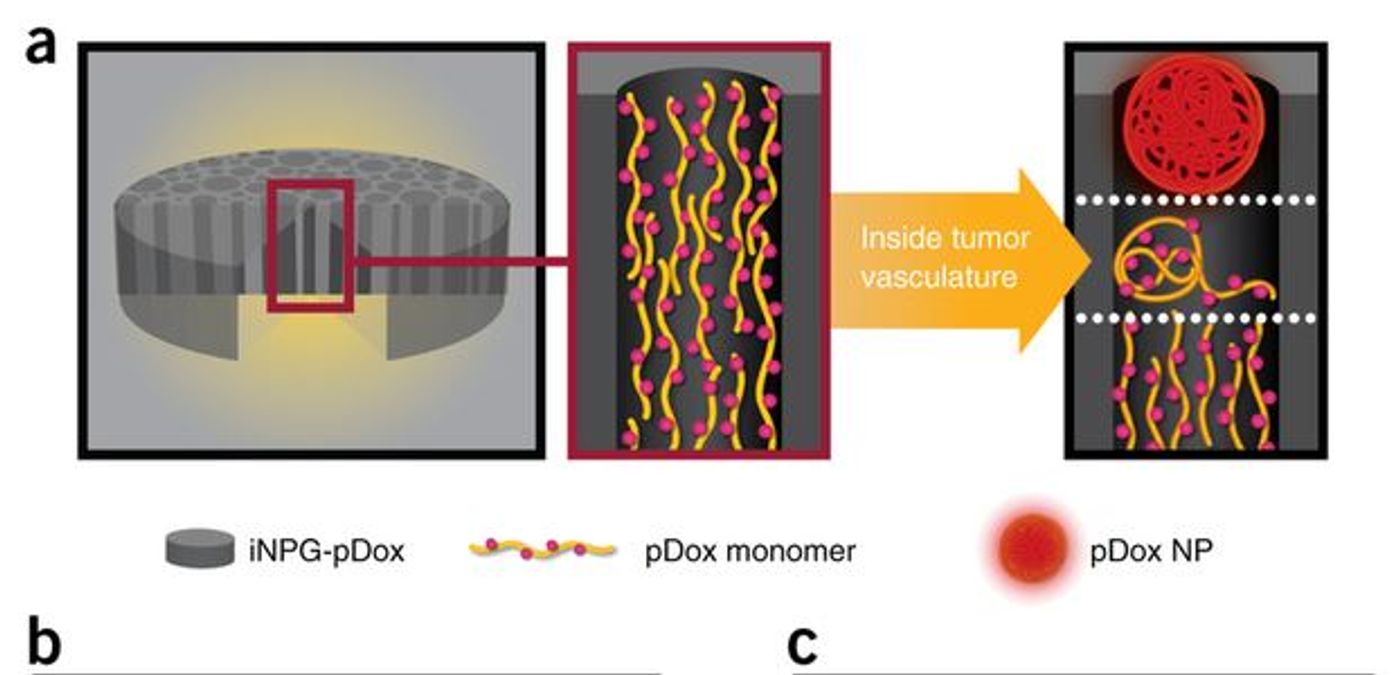
The construct they created, called an injectable nanoparticle generator (iNPG), is a silicon disk measuring 2.5 μm in diameter and 700 nm thick. The disc has pores throughout, ranging in size from 40 to 80 nm wide. The drug itself is loaded into the pores and is targeted to the tumors by the natural tropism of the discs. That means that the discs naturally accumulate at tumors. The drug that is loaded on the iNPG is not free drug; it is conjugated to poly(L-glutamic acid) by a pH-sensitive linker region. The drug used throughout the paper, doxorubicin (Dox), is a type of anthracycline, a therapeutic used to treat triple-negative breast cancer. The conjugates of multiple Dox molecules to poly(L-glutamic acid) is called pDox. The pDox is what is stored in the pores of the iNPG and it also plays a huge role in the kinetics and efficacy of this drug delivery platform. The entire complex, iNPG-pDox, is injected intravenously and in multiple models and on multiple measures, is more effective and safer than other formulations of Dox.
There are a few issues with cancer drug delivery that iNPG-pDox overcomes. First of all is targeting the drug to the tumor cells, which is solved by the natural tropism of the iNPG. Second is making sure there is high enough accumulation of the drug in the tumor to be effective. That is solved by the pDox. Once the iNPG-pDox construct has been localized near the tumor, it releases pDox as nanoparticles of various sizes. The pDox nanoparticles are taken up by the tumor cells through clathrin- and caveolae-mediated pathways and are transported deep into the cell to the perinuclear region. From there, the pDox is cleaved at the pH-sensitive linker into Dox by the acidic pH of the endosomes. Because the pDox was transported so deep into the cell, the free Dox is safe from the cell membrane drug efflux pumps and is able to effectively kill the tumor cells.
The iNPG-pDox was tested in two mouse models of triple-negative metastatic breast cancer against other formulations of Dox. iNPG-pDox treatment extended survivability of the mice more than any of the other treatments while also being much safer than free Dox. The iNPG-pDox complex actually remained in the tissue for a week, continually releasing pDox nanoparticles. In addition to the in vivo testing, the researchers tested the pDox nanoparticles in a cell model of multidrug resistant breast cancer that overexpresses the drug efflux pump P-gp. The multidrug resistant cell line was, expectedly, resistant to free Dox, but the pDox was just as effective at killing the multidrug resistant cancer cells as it was at killing the nonresistant cancer cells.
The results are very encouraging and this is the first time that anyone has created an IV drug that is capable of targeted, sustained in situ drug release. While the experiments done in this paper only used Dox and focused on triple-negative metastatic breast cancer, this construct could be applied to all different kinds of cancer. From a pharmacological perspective, this is a great example of rationally designing a drug delivery system. The researchers combined multiple methods to overcome the multiple problems in targeting tumor cells. Here’s hoping this technology is utilized as much as possible.
Source:
Nature Biotechnology