Protein quality control (PQC) is incredibly important for cellular health and viability. It is critical for cell survival that any protein that is no longer needed or somehow becomes misfolded is properly disposed of. If there is a buildup of misfolded proteins in the cell, the misfolded proteins can aggregate and impair other cellular processes and they can also grab other functional proteins and inactivate them. Because PQC is such an important factor in cellular health, there are multiple pathways dedicated to handling the problem of quality control. Misfolded proteins can be sequestered from other proteins in the cell, can be refolded properly with the assistance of chaperone proteins, or, if the misfolded proteins have aggregated, chaperone proteins can break up the aggregates. But the most important PQC mechanism is the ubiquitin-proteasome pathway. Misfolded proteins are tagged with long chains of ubiquitin which targets the protein-ubiquitin to the proteasome for degradation.
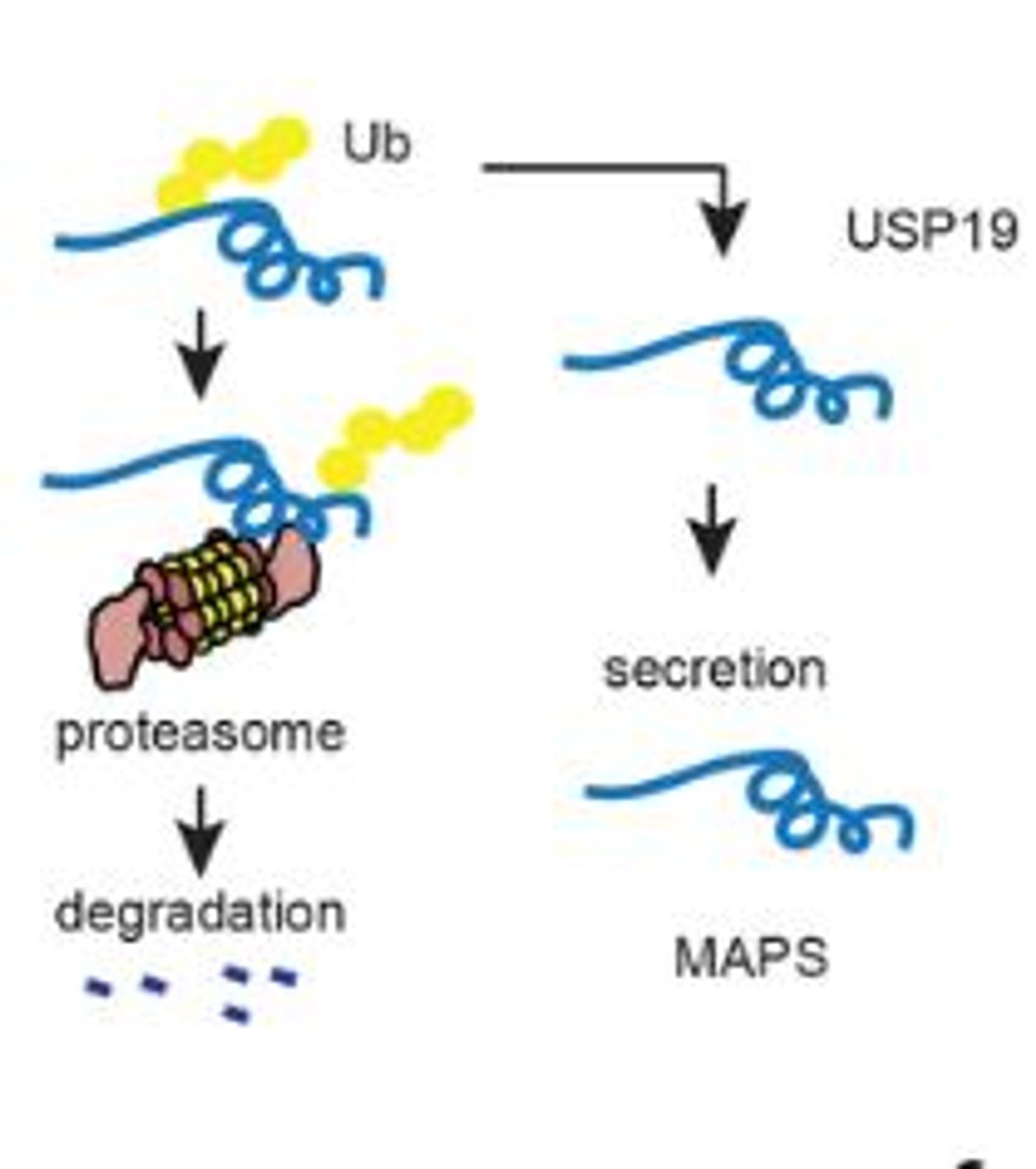
The ubiquitin-proteasome pathway requires many different types of enzymes and one such enzyme is the class of deubiquitylases which remove ubiquitin from proteins. The human genome encodes ~100 different deubiquitylases, but only one of these enzymes is localized to the endoplasmic reticulum (ER) membrane. This special deubiquitylase, USP19, is the key to a novel protein quality control pathway called misfolding-associated protein secretion (MAPS).
A recent, very thorough paper published in
Nature Cell Biology details this new, previously unheard of mechanism. The MAPS pathway is specific for misfolded proteins and also starts to provide an explanation for the evidence that misfolded proteins in neurodegenerative diseases spread from neuron to neuron in a prion-like manner.
USP19 is a deubiquitylase that is anchored in the ER membrane. >99% of late endosomes form a tight association with the ER membrane as well. In the MAPS pathway, USP19 specifically recruits misfolded proteins with defective ubiquitin signals to the ER membrane where it removes the defective ubiquitin chain and facilitates the transfer of the misfolded protein cargo to the late endosome. The late endosome then moves up to the cell membrane, fuses with the membrane, and releases its cargo of misfolded proteins into the extracellular space.
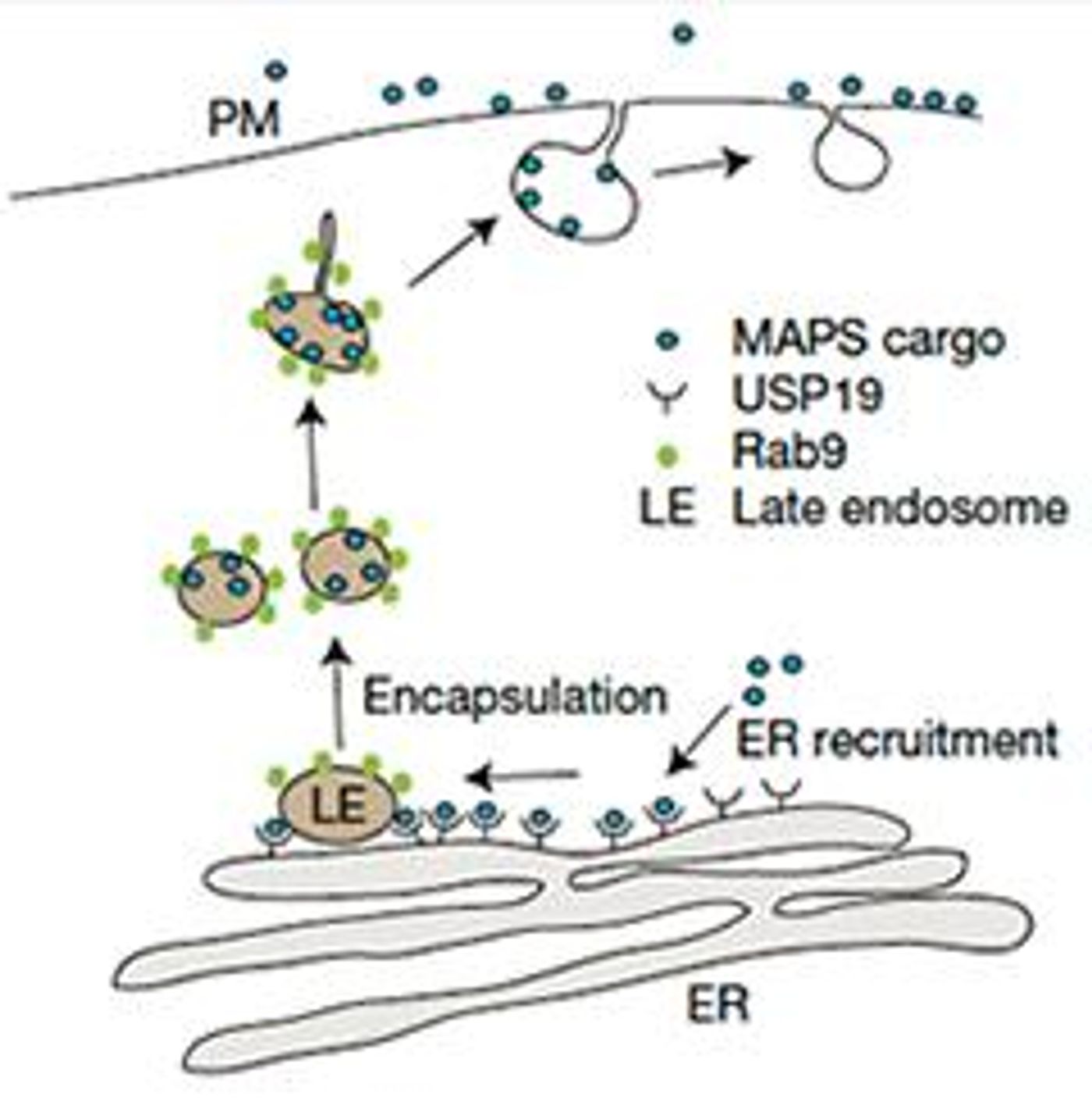
The researchers, lead by Yihong Ye from the NIH in Bethesda, performed most of their experiments using human embryonic kidney (HEK) cells and green fluorescent protein (GFP), but there is a lot of excitement about how the MAPS pathway applies to neurodegeneration.
Specifically, proteasome dysfunction is an early sign of neurodegenerative diseases and the MAPS pathway really comes into play when the proteasome system is compromised. The theory is the the buildup of misfolded proteins over many, many years puts strain on the ubiquitin-proteasome system, pushing more misfolded proteins to be excreted through the MAPS pathway. Once the proteins are secreted, they can be taken up by other neurons, propagating the spread of these misfolded proteins.
This really is a theory because the MAPS pathway has not been investigated in neurons yet. However, the researchers wanted to get the ball rolling on this line of inquiry so they looked at α-synuclein and the MAPS pathway. They showed that, in HEK cells, misfolded α-synuclein with known Parkinson’s disease mutations is secreted from cells through the MAPS pathway. That means that the MAPS pathway, should it translates to neurons, could be a potential therapeutic target for Parkinson’s disease.
Sources:
AlzForum and
Nature Cell Biology