PLSCR1 Inhibition Tamps Down the Brain's Immune Response
In the central nervous system, cells called microglia act to protect it. When a pathogen like a virus activates microglia, they can reduce damage to tissue and halt the spread of the threat. However, that activation must be carefully controlled because it can also be a factor in cell death and disease. It’s important to learn more about that response for a variety of reasons; not only do viruses themselves pose a danger, some therapeutics use viruses in treatment, and controlling the immune response to viruses is essential for the success of those treatments. Researchers at the Salk Institute in La Jolla, California have now made a major step forward in that understanding. The video below explains more, and their work was published in the journal Neuron.
The investigators found that the phospholipid scramblase 1 (PLSCR1) protein regulates the response of a cell to a virus and when inhibited, acts to stop excessive inflammation while conferring protection from immune attack for the long-term. This work could influence therapeutics for autoimmune disorders, neurodegenerative diseases, and therapies utilizing viral modes of delivery.
"Normally, the immune system will quickly recognize and act upon potential threats such as virally infected cells," explained the senior author of the study, Axel Nimmerjahn, an Assistant Professor in Salk's Waitt Advanced Biophotonics Center. "But in targeting PLSCR1, we've effectively shielded infected cells from immune attack and increased gene expression from an engineered virus from a few days or weeks to at least six months, creating the potential for much longer-lasting therapies."
Viruses are masters at zeroing in on a target cell and taking over the machinery inside to meet its own ends. Scientists seeking a way to deliver drugs to a specific cellular target or destroy a tumor cell while leaving healthy cells unharmed thus knew that modified viruses could be a way to achieve that goal. Immune systems however, can’t tell the difference between a virus meant to heal and one that is harmful; the immune response must be controlled if viral therapies will succeed.
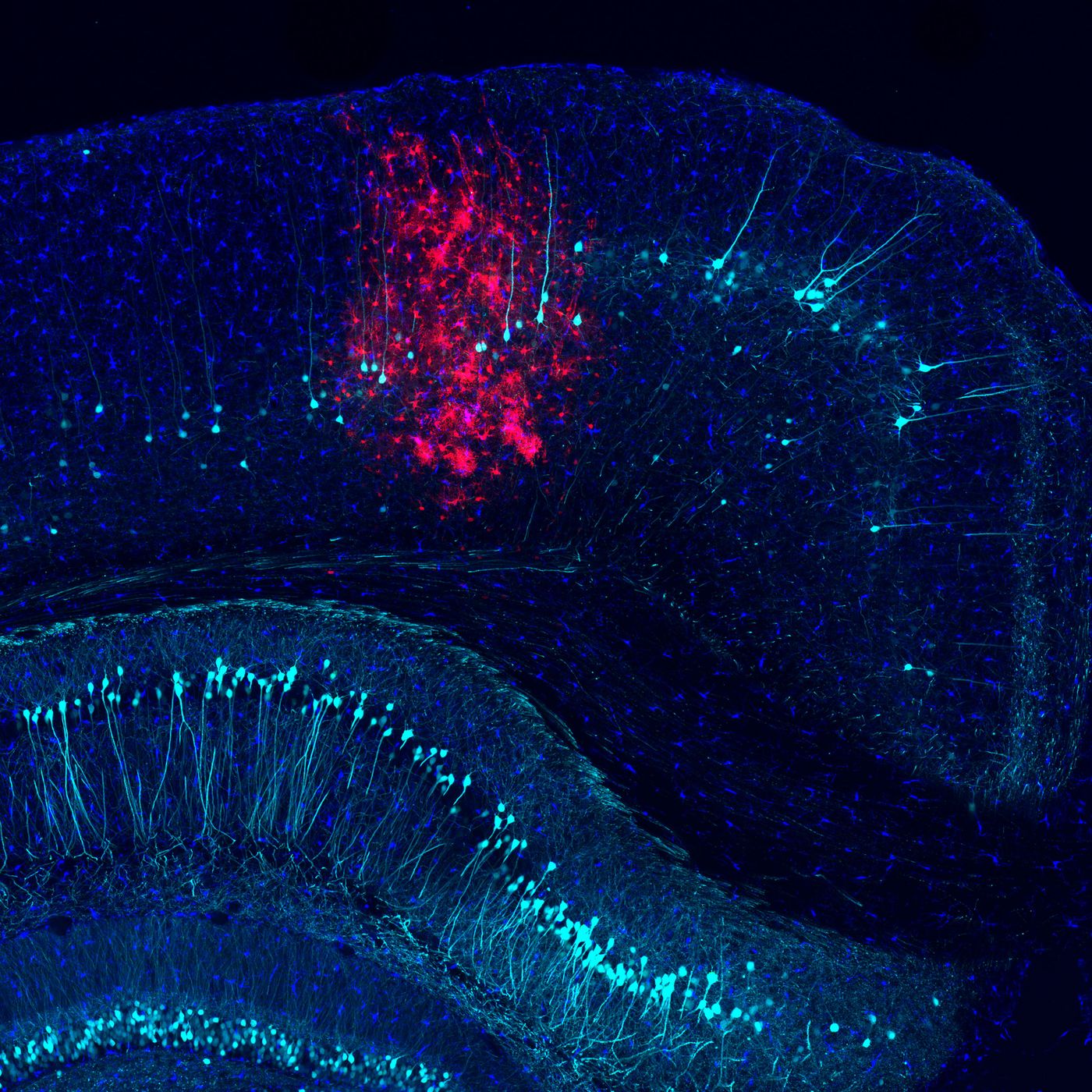
An adenovirus, like one used in viral therapies, was injected into mouse brains so the scientists could understand the immune response and learn more about the molecular response to an infection. Microglia act in that response, beckoned to the site of an infection in the brain by a signal called phosphatidylserine. The miocroglia then assess the situation, either destroying the infected cells, or leaving them alone. When a brain cell is destroyed, it may not regenerate; that decision must be made with care. However, an infected cell also presents a danger. To find out more about what influences that choice in microglia, the scientists altered the levels of proteins that act in intracellular communication, including PLSCR1.
Manipulating the levels of PLSCR1 resulted in many changes to immunity mechanisms. Lower levels of PLSCR1 lowered cytokine production as well. Since cytokines act to ramp up the immune response, lower levels of cytokines tamps the immune response down even more.
"When we saw how much inhibiting PLSCR1 reduced the inflammatory response, we immediately wondered if this mechanism could apply more broadly, not just to virus infection of the brain, but to other types of infections or even autoimmune diseases," explained the first author of the work, Yusuf Tufail, a former Salk postdoctoral fellow.
Inhibition of PLSCR1 continued to exert control over the immune response for up to six months, the best result for any protein tested by the researchers. PLSCR1 is not only located in the brain, so researchers are working to learn more about its role in other types of inflammation.
"Given how complex the immune response is, and how many genes are regulated up or down in response to infection, it was amazing to find a single protein that controls so many pathways," said Nimmerjahn. "Imagine a small molecule inhibitor that a patient could take to curb excessive inflammation. This could have a hugely beneficial effect on many disease outcomes."
Sources: AAAS/Eurekalert! via Salk Institute, Neuron