Preliminary Approval Issued for Personalized Cancer Therapy
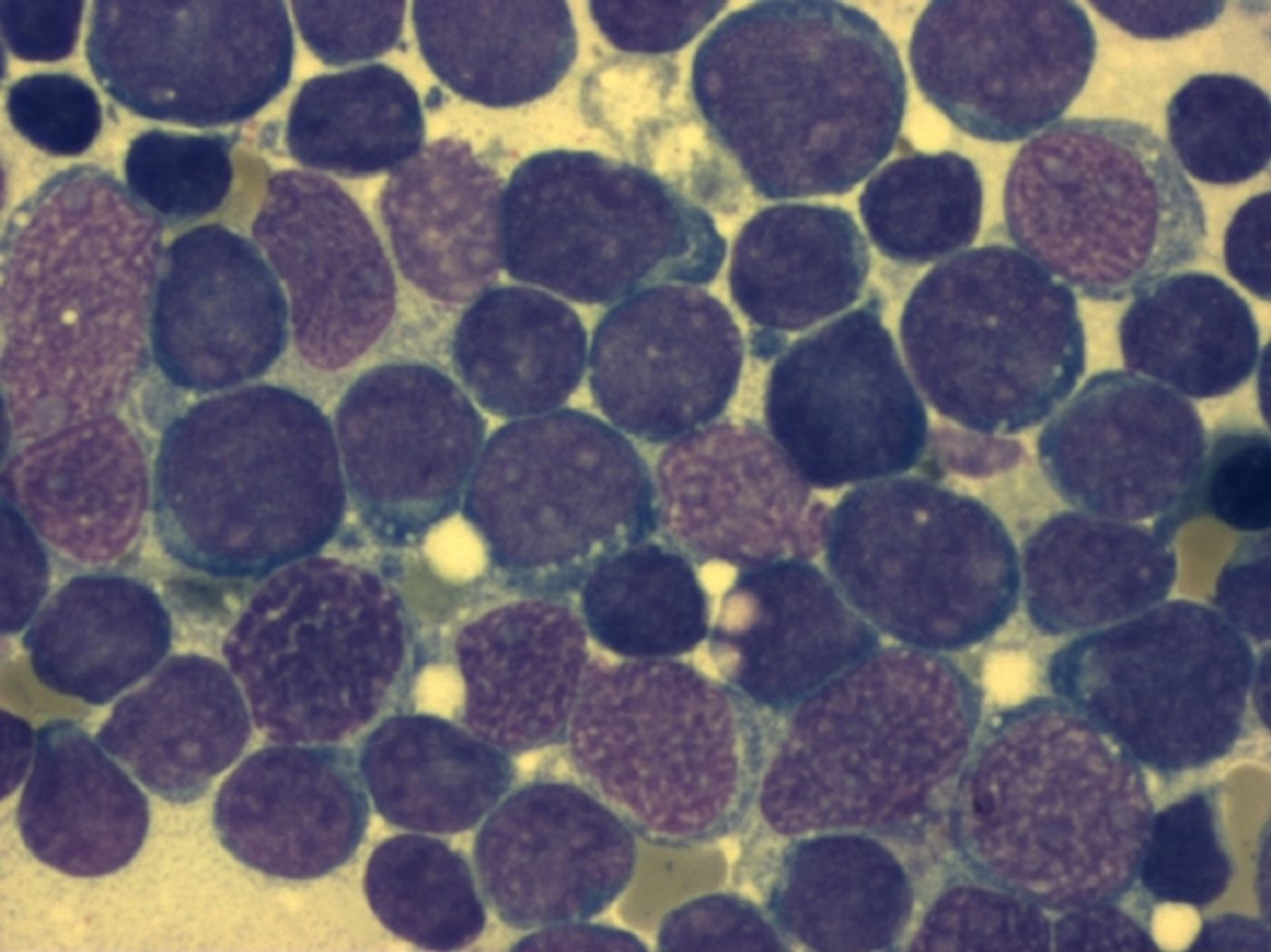
A committee of experts appointed by the Food and Drug Administration (FDA) has approved an experimental personalized therapy, in a first for the United States. In a clinical trial of patients suffering from severe cases of acute lymphoblastic leukemia (ALL), 83 percent of them were free of cancer after three months. Final approval, expected from the FDA on October 3, could be life-changing for those suffering from ALL.
The treatment, called CTL019, harvests a patient’s white blood cells and genetically reprograms them so that they will become cancer-fighting machines. Specifically, after being removed from the patient, T cells are engineered to express a receptor that will recognize a particular antigen that is indicative of cancerous cells. A patient's natural defense mechanisms can now be used against cancer.
"The panel's unanimous recommendation in favor of CTL019 moves us closer to potentially delivering the first-ever commercially approved CAR-T cell therapy to patients in need," said Bruno Strigini, CEO of Novartis Oncology. "We're very proud to be expanding new frontiers in cancer treatment by advancing immunocellular therapy for children and young adults with r/r B-cell ALL and other critically ill patients who have limited options. We look forward to working with the FDA as they complete their review."
This particular treatment was developed by Novartis, although other companies are making their forays into this area. Kita Pharma is awaiting their own approval decision on November 29. The video below explains more about this milestone.
Novartis worked with Penn University to create this treatment, which could be a new avenue for kids facing limited options. ALL is responsible for around 25 percent of all childhood cancer diagnoses. For patients that experience multiple relapses, the five-year disease-free survival rate drops to around 20 percent.
"It is encouraging to see the FDA panel's recommendation and continued momentum behind this innovative therapy, which has potential to help young patients with relapsed/refractory B-cell ALL," said the Penn team's leader, Carl June, MD, the Richard W. Vague Professor of Immunotherapy, Director of the Center for Cellular Immunotherapies in Penn's Perelman School of Medicine and Director of the Parker Institute for Cancer Immunotherapy at Penn. "We look forward to continuing to work with Novartis to help make a lasting impact on the way this disease is treated."
"We know firsthand from treating children and young adults with relapsed/refractory B-cell ALL that they desperately need innovative medicines that provide a new approach to managing this aggressive disease," said Stephan Grupp, MD, PhD, the Yetta Deitch Novotny Professor of Pediatrics at the Perelman School of Medicine at Penn, Director of the Cancer Immunotherapy Frontier Program and Chief of the Section of Cellular Therapy and Transplant at CHOP. "Today's vote in favor of CTL019 is a positive step, and we appreciate Novartis' commitment to pediatric patients."