New Multiple Sclerosis Drug Wins FDA Approval
The FDA recently approved the first drug for the treatment of relapsing forms of multiple sclerosis (MS). The announcement marks a significant achievement, which is expected to help the 400,000 MS patients in the US.
Multiple sclerosis is described as a demyelinating disease whereby the body’s immune system mistakenly attacks the myelin sheath covers in the brain and spinal cord. This disrupts the electrical signal traveling from the brain to the body, and leads to physical and neurologic disabilities. The progressive form of MS causes patients to slowly deteriorate over time, while the relapsing form causes patients to go through bouts of symptoms and recovery. There is no cure for either form of MS.
“Multiple sclerosis can have a profound impact on a person’s life,” said Billy Dunn, the director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. “This therapy not only provides another treatment option for those with relapsing MS, but for the first time provides an approved therapy for those with primary progressive MS.”
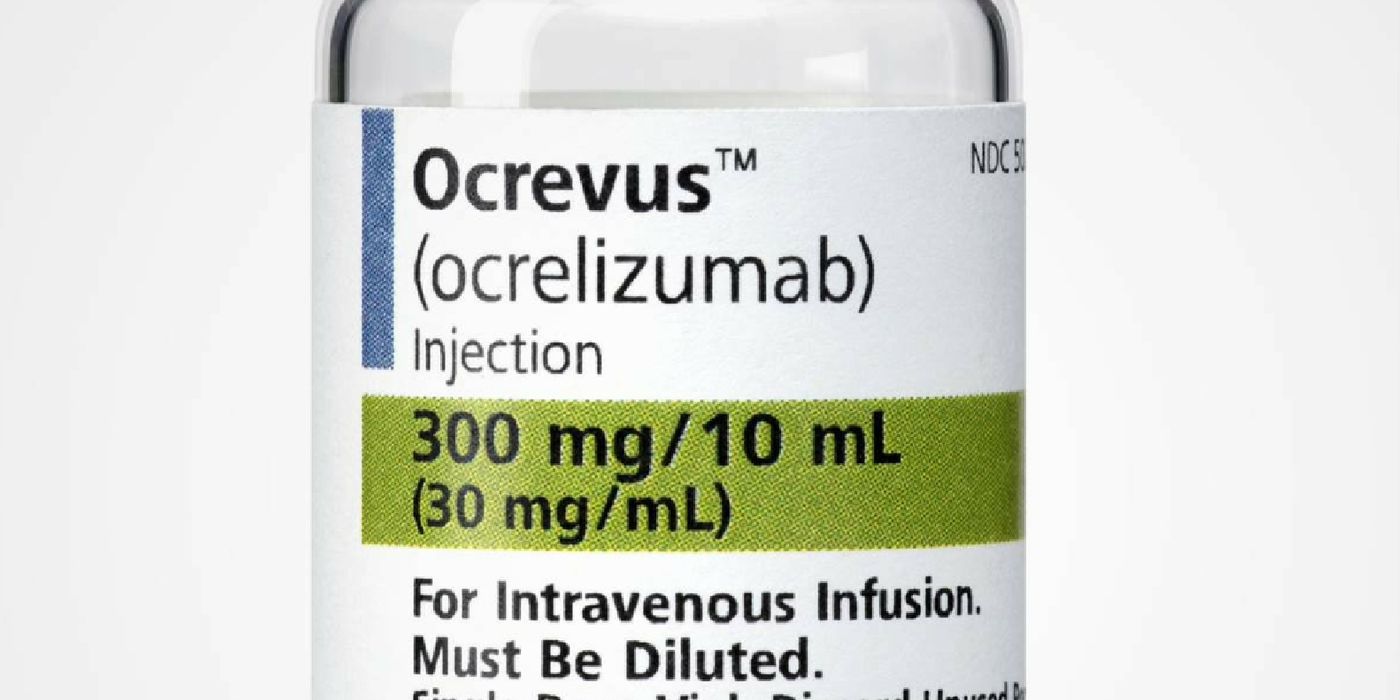
The drug is known on the market as Ocrevus (ocrelizumab), and it works by weakening the immune system. In particular, IV infusions of Ocrevus prevent the B cells from attacking the protective myelin covers.
Tested in patients with both forms of MS (the progressive form and the relapsing remitting form), Ocrevus seems to stop the inflammation process in the brain. This results in less fatigue and muscle weakness. Vision seems to also be improved. Furthermore, scans show significantly less brain loss with the help of ocrelizumab.
"The drug is so much more effective at shutting down inflammation," said David Hafler, an MS expert from the Yale School of Medicine.
Side effects associated with Ocrevus include infusion-related reactions, such as skin irritation, flushing, shortness of breath, and nausea. In addition, upper respiratory tract infections were among the most common side effect seen with the drug in both forms of MS.
Patients eager to be treated with Ocrevus will have to wait just a few more weeks. Furthermore, they should also be aware that this drug comes with a hefty price tag of $65,000 per year. Though the cost is substantial, it is reportedly on par with approved drugs for the other forms of MS.
Additional sources: CNN