Unique Epigenetic Signatures Diagnose Rare Disorders
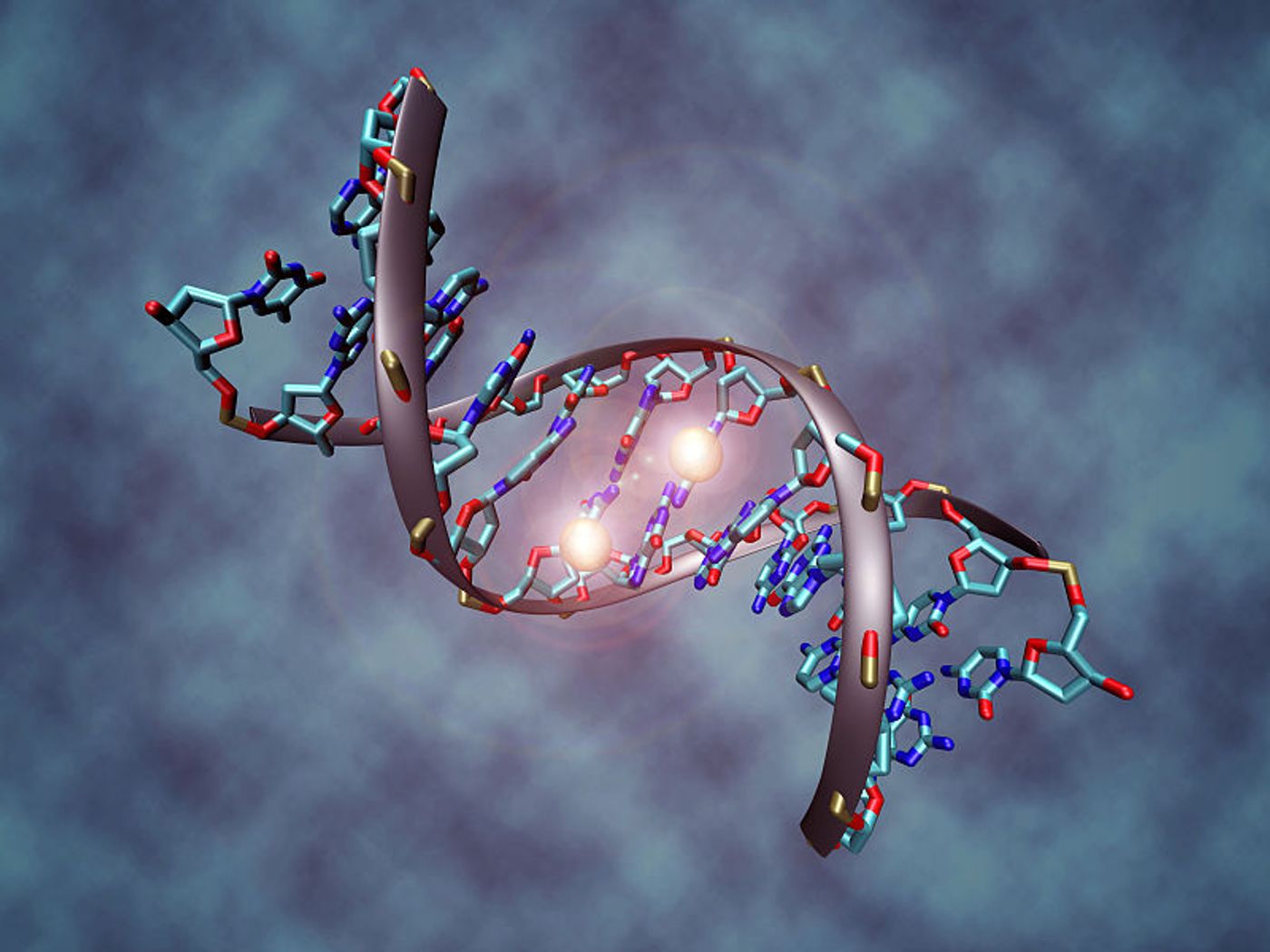
Epigenetic changes impact gene activity without actually altering the sequence of DNA, and scientists are now able to diagnose rare disorders based on specific epigenetic signatures. From the Greenwood Genetic Center (GGC), scientists are improving diagnosis of neurodevelopmental disorders that are often difficult to tell apart.
"Many of the disorders we studied have clinical overlap, which makes sense as the genes causing each of these disorders all work within the same epigenetic machinery, determining the gene's expression and expression of other genes," explained co-author Charles Schwartz, PhD.
But in a new study of 14 neurodevelopmental disorders, Schwartz and other GGC researchers identified nine of the disorders as having unique methylation signatures. DNA methylation is a known process of epigenetics, where a methyl group is either added or removed to change DNA expression. The research team was able to produce this finding using a methylation array analysis. Of the rare disorders included in this study, Sotos syndrome, Kabuki syndrome, CHARGE syndrome, and ATRX were among those with unique epigenetic signatures.
Applying each of the signatures associated with the disorders to existing tests could greatly improve the accuracy of diagnoses. Scientists could screen for multiple symptoms with maximum sensitivity and specificity. Additionally, they could build a “single machine learning classification tool” to make accurate diagnoses.
Most important is using the unique epigenetic sequences to differentiate between each of the nine disorders. For patients whose results show no sign of any of the nine disorders, researchers could also assign “low probability scores,” providing the patients additional information of their risk of developing these disorders.
Lastly, facilitating these tests could be faster and more efficient, with no need for parental samples or brain tissue to test; peripheral blood samples from each patient will suffice.
Schwartz says that the findings also produced an “epigenetic echo model” that illustrates additional information about each disorder’s unique epigenetic signature: what causes them to develop and cause disease.
"The echo model states that while an initial epigenetic dysfunction arises during early development, a broader methylation legacy or echo remains throughout the genome leading to the unique signature,” Schwartz explained. “This may help explain the variability and phenotypic overlap among these disorders."
The present study was published in the journal American Journal of Human Genetics.
Sources: Environmental Health Perspectives, Greenwood Genetic Center