Chronic Infections Outsmart the Immune System
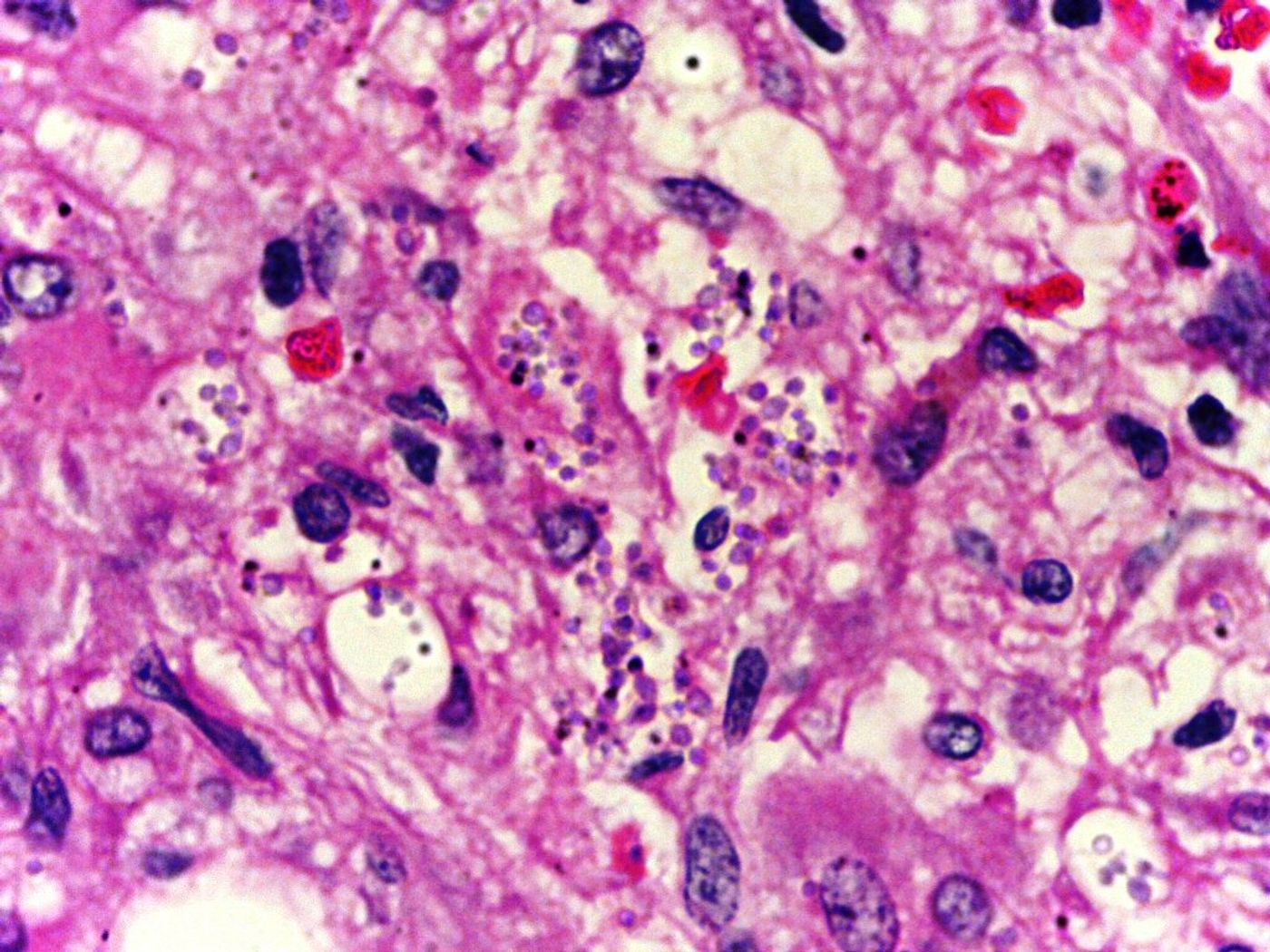
Many chronic infections evade the immune system’s defenses so that it can remain comfortably in the host mooching off our bodies. A recent study in Cell Reports looked at how the parasite that causes visceral leishmaniasis, also known as kala-azar or black fever, escapes the host’s defenses. Visceral leishmaniasis, a potentially lethal chronic disease, is the second leading cause of death by parasitic infection. It is caused by the Leishmania species of protozoan parasite and transmitted to humans by the bites of female sandflies. In 2015 the World Health Organization (WHO) reported 300,000 estimated annual cases with over 20,000 deaths each year due to visceral leishmaniasis. Of these cases, 90% were reported to occur in six countries: Bangladesh, Brazil, Ethiopia, India, South Sudan and Sudan. Onset can be chronic or acute, with incubation of weeks to months, and it affects internal organs such as the spleen, liver, and bone marrow.
Interferon-gamma, or type II interferon, is a cytokine essential to innate and adaptive immunity against infections. Interferon-gamma has immunostimulatory and immunomodulatory effects and is produced by natural killer cells in the innate response and T cells CD4 and CD8 during the adaptive immune response. During visceral leishmaniasis, CD4 T cells expressing interferon-gamma appear later in infection and in smaller numbers than expected, but the recent study by Dr. Simona Stager’s team at the Institute National de la Recherche Scientifique (INRS) has shown that the lack of T cells is due to the protective cells dying. To further study this phenomenon they looked at previously identified transcription factors, proteins that modulate the activity of one or more genes, that were present but unusual for CD4 T cells. The specific transcription factor detected, IRF-5, is known for its role in innate immunity but not CD4 T cells.
The study of IRF-5 showed that it leads CD4 cells to self-destruct due to tissue destruction. Previously unknown signals in CD4 cells are activated when tissue is destroyed, causing them to die following a series of reactions that are not fully understood. The mechanism behind this signaling begins with Toll-like Receptor 7 (TLR7), which is usually activated in innate immune cells when a pathogen is recognized. During chronic inflammation cellular residues present after tissue destruction activates TLR7 in CD4 cells. This activation leads to expression of IRF-5, which then increases expression of Death Receptor 5 (DR5) and caspase 8, which leads to cell death. The white blood cells involved in the attack are eliminated by the biological process of the host and not the parasite, allowing the parasite to be protected and sustain a chronic infection.
This mechanism may play a role with other diseases that lead to chronic infection and inflammation. The newly discovered link between chronic inflammation induction of CD4 T cell death by the TLR7 and IRF-5 pathways could lead to new treatment options. A better understanding of this work and the cellular activity induced by IRF-5 in immune cells could reveal new therapeutic targets for visceral leishmaniasis and other chronic inflammatory diseases.
To learn more about leishmaniasis watch the video below!