Reversing T Cell dysfunction in Cancer: Getting the Body to Fight its own Battles
Using the power of the immune system to eliminate cancerous cells has long the been the goal of immuno-oncology. Immune cells naturally recognize, destroy, and remember the abnormal cells within our bodies while also overlooking healthy cells. This process is called self-tolerance, and when working well, allows the body to rid itself of abnormalities without collateral damage. The challenge with cancer is that tumors can co-opt a variety of self-tolerance mechanisms to evade the destructive potential of the immune system.
Self tolerance prevents the immune system from becoming overactive and protects against autoimmunity. Autoreactive immune cells are either destroyed or left inactive. For example, circulating T cells are prevented from excessive proliferation and cytotoxicity by signaling molecules expressed on tissue and regulatory T cells with suppressive functions called Tregs. Tumors take advantage of this self tolerance in order to grow unchecked. A comprehensive approach to reversing this dysfunction by (1) activating cancer-specific cytotoxic T cells, (2) releasing T reg suppression, and (3) producing tumor-specific memory T cells has been termed the immune checkpoint blockade.
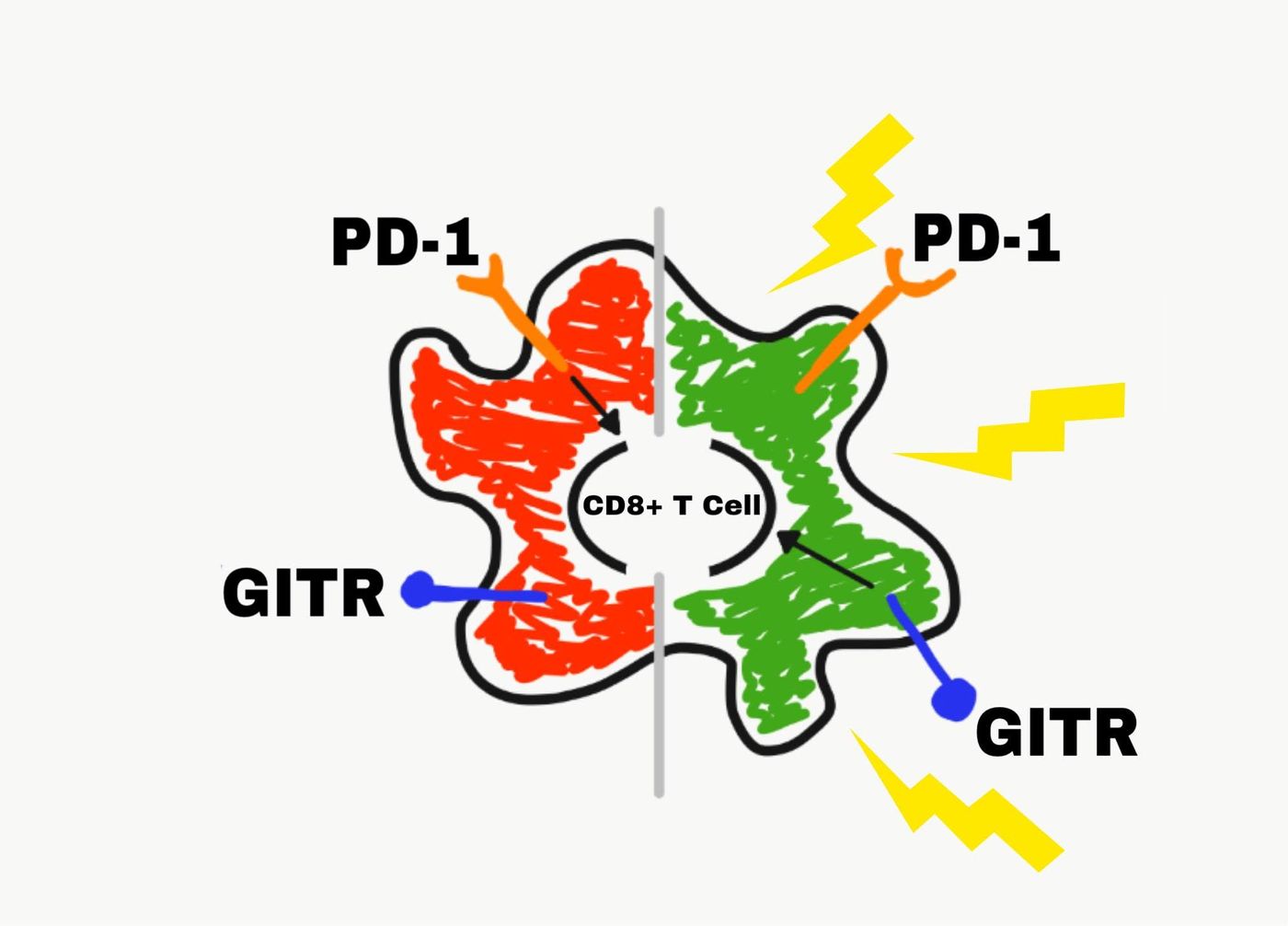
Current attempts to achieve the immune checkpoint blockade have focused on targeting signaling molecules involved in maintaining self tolerance. Two such proteins are PD-1 and GITR, and both are frequently exploited by tumors. PD-1 is a protein that acts as a “brake” for the immune system. It is expressed on immune cells including T cells and T regs. Its ligand is expressed on both healthy tissue and tumors. PD-1 signaling inhibits the activity of cytotoxic T cells while promoting the expansion of Tregs.
GITR is a cell surface receptor that acts as an “accelerator” for the immune system. Like PD-1, GITR is found on activated T cells and Tregs, but it has the opposite function. GITR signaling promotes cytotoxic T cell expansion and inhibits Treg activity.
Initially, scientists attempted to activate anti-tumor immunity by either inhibiting PD-1 signaling (cutting the “brakes”) or activating GITR signaling (revving the “accelerator”). Neither indvidual approach was able to achieve immune checkpoint blockade. However, a combinatorial method using an antibody to block PD-1 signaling (anti-PD-1) while simultaneously activating GITR signaling by antibody (anti-GITR) has shown promise.
“In preclinical studies in which monotherapy with anti-GITR or anti–PD-1 Ab has limited efficacy… combination therapy was able to achieve long-term survival in mouse models of ovarian and breast cancer”
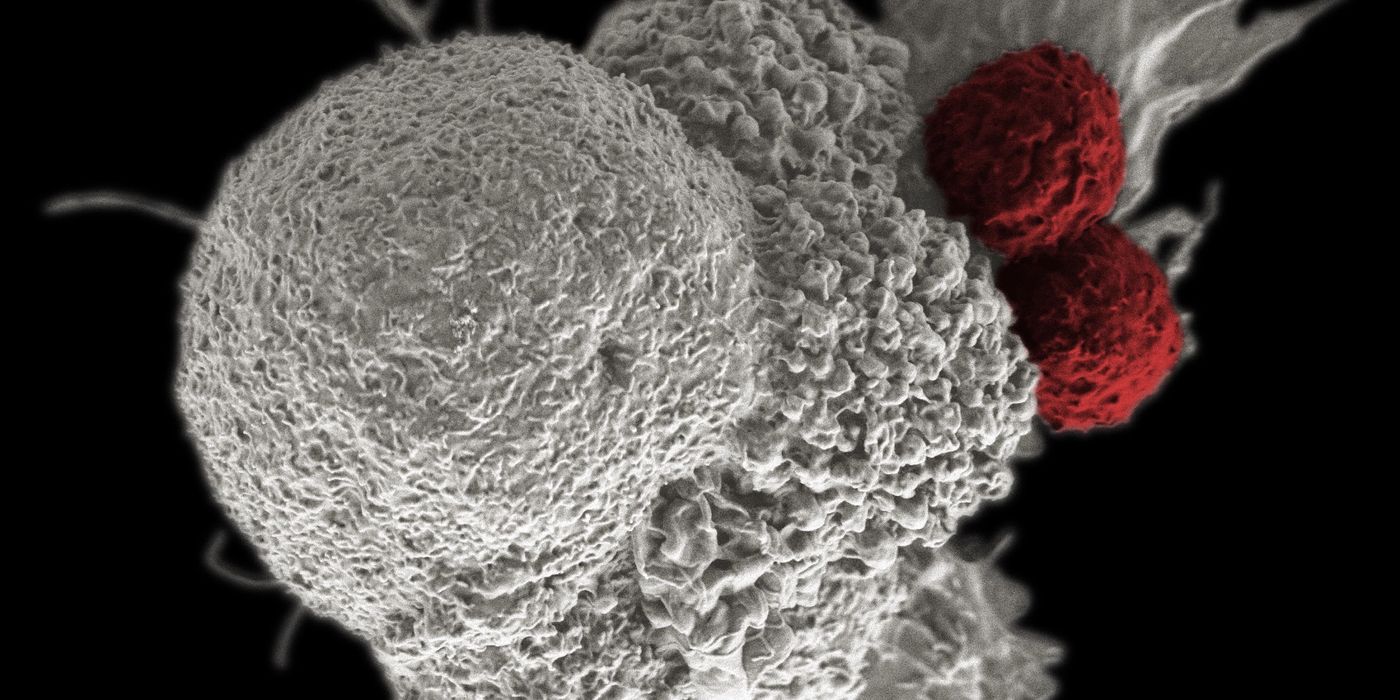
To study this anti-tumor treatment, Bei Wang and others at Regeneron Pharmaceuticals used a murine colon adenocarcinoma model to analyze populations of intratumoral cytotoxic CD8+ T cells and Tregs following treatment with anti-PD-1 and/or anti-GITR antibodies. Monotherapy with either anti-PD-1 or anti-GITR were not capable of controlling tumor growth as neither could overcome the immune checkpoint. However, the combination therapy resulted in the rejection of the tumors along with in an increased ratio of T cells to Tregs. Further studies showed that tumor rejection was CD8+ T cell dependent, cancer specific, and that the CD8+ T cells contained memory cell precursors suggesting anti-tumor memory.
The higher numbers of functional CD8+ T cells compared to Tregs along with the rejection of the tumors and presence of memory cell precursors after the combination treatment suggests that the immune checkpoint blockade was successful. Excitingly, this therapy does not appear to be cancer type specific as anti-PD-1 + anti-GITR treatment also resulted in rejection of a mouse model of kidney carcinoma.
It will be interesting to see whether this combination treatment, with or without cancer vaccination or chemotherapy, has the same dramatic results in humans.
Sources: European Journal of Cancer; Science Immunology; Johns Hopkins Medicine;