FDA approves first off the shelf DNA testing for breast cancer risk
Most people must have heard or used the 23andMe company for its ancestry genetic testing, but now it is going into off the shelf clinical diagnostic service. Recently, FDA approved company's kit for screening breast cancer risk which will be offered to the consumer without doctor’s prescription. However, people should not be overwhelmed to get tested due to the limitation of the test. The test screen for only three mutations associated with BRCA-1 and-2 gene among thousand known mutations. Moreover, tested variations are more associated with the increase in breast cancer risk in the Ashkenazi Jewish population.
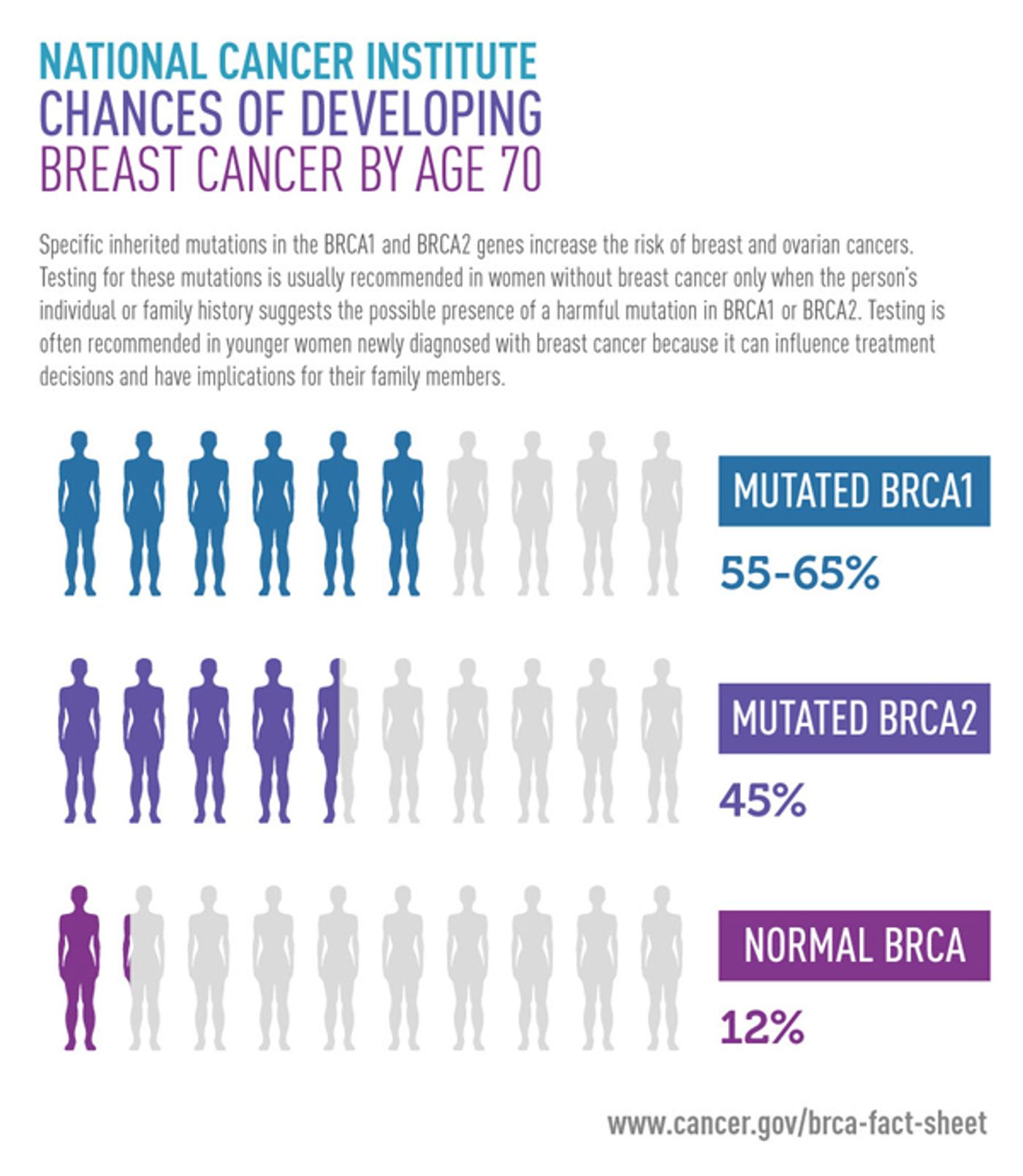
BRCA-1 and-2 are unrelated genes mostly expressed by all the cells with increased abundance in breast tissue. These two genes are responsible for DNA repair mechanism, and any mutation in these genes may result in either cell death or unchecked cell proliferation. There are thousands of known BRCA-1 and-2 mutations cataloged, but not some of them are not associated with cancer. Some mutations increase cancer risk in a specific ethnic group while others are not known whether they are risky in an individual. A harmful variant of BRCA gene is dominant and thus can easily transfer breast cancer risk from parents to children. It is very common to screen for these detrimental variants of BRCA gene in a suspected individual with the history of breast, ovarian, prostate and few other cancers in family members. These BRCA mutation screening test are prescribed by certified doctors and are expensive in nature. However, recently FDA approved an off the shelf diagnostic test through 23andMe genetic ancestry screening company. This test will not require any prescription from doctors. The company spokesperson told that screening covers variants which result in the highest risk of developing cancer among women and men. The company spokesperson also underscores the point that negative result does not rule out other mutations in BRCA genes, which are more prevalent among breast cancer patients. This test will be more useful for people with Ashkenazi Jewish ancestry as their chance of having BRCA mutations are 1 in 40 in comparison to 1 out of 400 among the general population. Moreover, among Ashkenazi Jewish people, three tested variants of BRCA gene account for 90% of BRCA related cancer. Therefore, this test is for targeted for a specific ethnic group but their marketing team suggests that it is for the general population. A negative test could bring a false sense of security among the general public.
The 23andMe company was in hot water with FDA a few years back with its genetic screening service where it started telling people for increased risk for certain diseases based on the individual result. FDA did not approve this genetics risk assessment and, that lead to notice from the federal agency. The company did a lot of cross-validation and got final approval from FDA for its breast cancer risk assessment based on two mutations in BRCA-1 gene and one mutation in BRCA-2 gene. This test will not be available as a stand-alone test but will be part of ancestry plus disease risk screening kit.
Credit: Lisa Schwartz, MD