Self-deoxygenating Glassware: Sugar In, Oxygen Out
Oxygen is often the headache for chemists since its ubiquitous presence in our environment, and its ability oxidize sensitive compounds.
A team of chemistry researchers at the University of Melbourne recently reported that they have come up with an inexpensive, gentle glassware coating that helps to remove water-soluble oxygen in reactions.
Currently, there are a few different approaches chemists employ to remove oxygen in aqueous solution, including bubbling the liquid with inert gases and applying vacuum to the container(s). Often for air or humidity-sensitive chemicals, scientists need to handle them with argon-filled vessels and in high-vacuum glove boxes. These conventional methods are either time-consuming or expensive.
The Australian group was working on RAFT (short for reversible addition-fragmentation chain transfer) polymerisation reactions when the idea of self-deoxygenating glassware struck them.
RAFT: Creating better polymers to transform everyday products (CSIRO)
RAFT polymerization is a type of controlled polymerization technique that generates polymers with a large variety of sizes and shapes: they can be linear, branched, grafted, and shaped like a comb, bush or star. First developed by Australia's Commonwealth Scientific and Industrial Research Organisation (CSIRO), the chemical reaction is useful in creating tailered polymers with special properties. However, this polymerization method has an Achilles' heel. It cannot tolerate any amount of oxygen. Once oxygen sips into the reaction solution, it instantly terminates propagation.
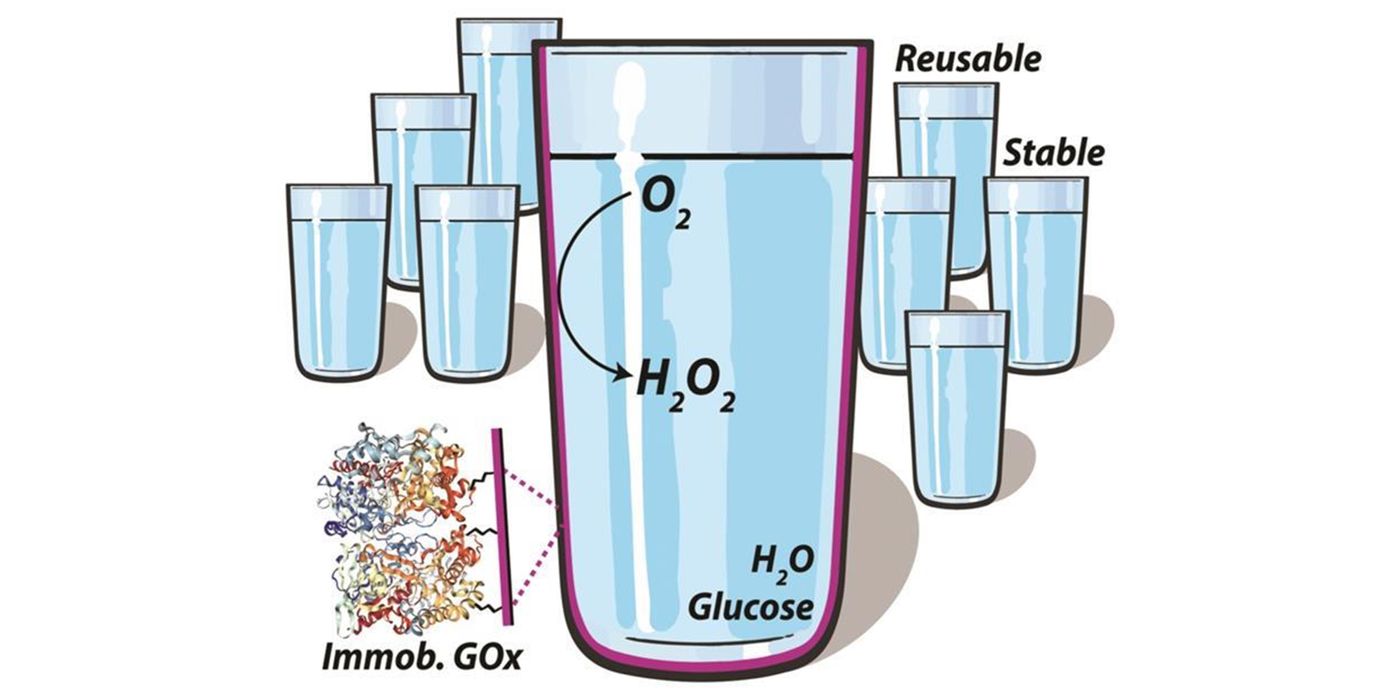
To effectively remove oxygen, the University of Melbourne team coated a variety of glassware with immobilized glucose oxidase. To activate the self-deoxygenation feature, one just needs to add glucose, which acts as a substrate to the oxidase. The process of glucose oxidation turns the oxygen dissolved in aqueous solutions into hydrogen peroxide, the chemical initiator that is needed for polymerization.
The researchers demonstrated the effectiveness of the coating by successfully conducting RAFT polymerizations without any other deoxygenation measures.
This latest research was published in the journal Chemical Communications.
Source: Chemistry World