Neuroscience
Noradrenergic Signaling Determines Resilience to Depression
FEB 16, 2016 11:36 AM PST
Share
Incremental Strides Made in Women's Health & Cancer Care

The changes are spurred by FDA-approval of a Human papillomavirus (HPV) test for primary screening, availability of the 9-valent HPV vaccine, and new recommendations for screening women living with human immunodeficiency virus (HIV), according to David Chelmow, MD, who steered development of ACOG’s January 2016 Practice Bulletin. There was also a need to reconcile some recommendations after other medical organizations updated their guidelines for managing abnormal test results.
Prior to the release of Practice Bulletin Number 157, the most recent bulletin on these topics (Number 131), was published in November 2012. Practice Bulletins are evidence-based documents that boil down prevailing knowledge about techniques and clinical management issues, which hinge on the evidence contained therein, according to ACOG. While ACOG has not altered its existing recommendations on the role of primary cervical cancer screening with HPV testing alone for women 25 and older, it did acknowledge a development within two fellow medical organizations for its own members.
“We continue to recommend Pap tests every three years for women 21 to 29, and co-testing every five years for women 30 and older,” Chelmow says. “As the FDA has approved the [Roche cobas] HPV test, we wanted to make sure our providers were aware of it, and that they followed the ASCCP/SGO interim guidance if they did choose to use the test.”
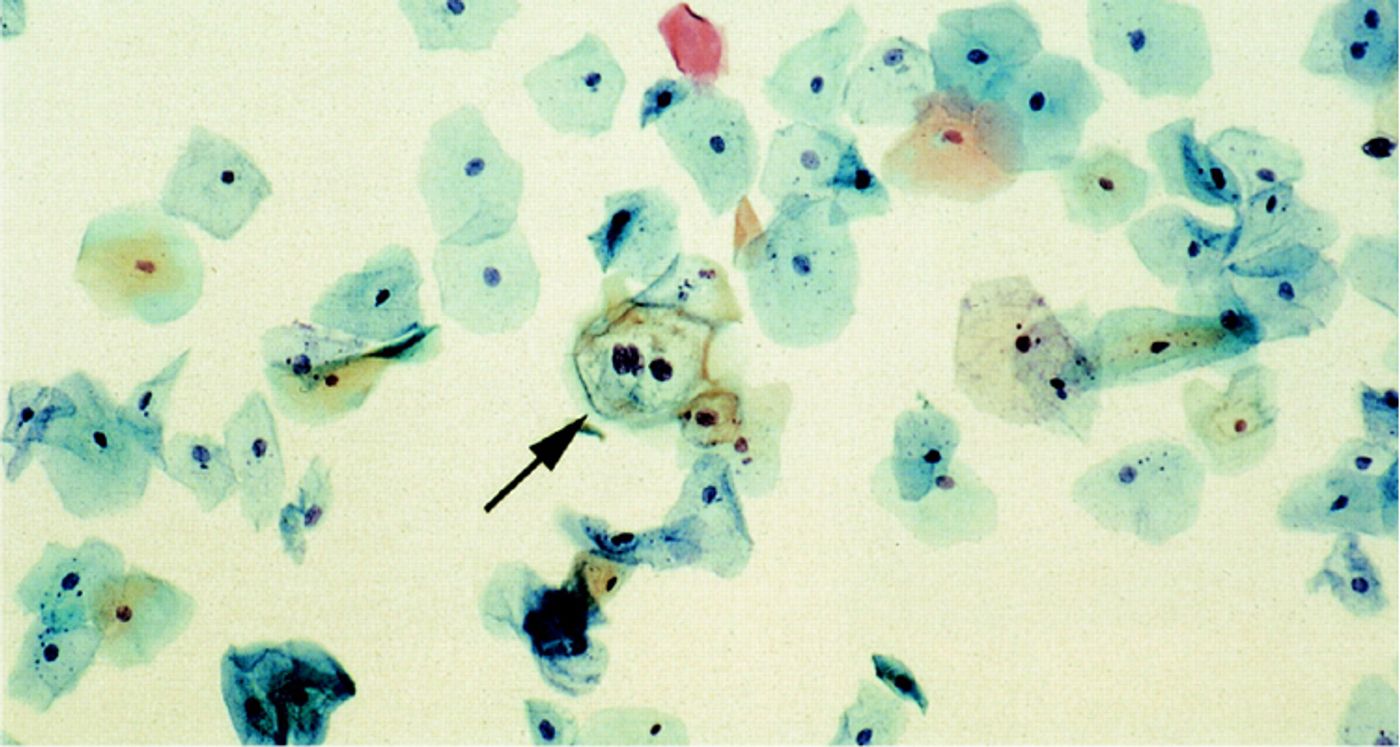
Infection with high-risk types of HPV is the root cause of most cases of cervical cancer.
The Interim Guidance Report issued jointly by the American Society for Colposcopy and Cervical Pathology (ASCCP) and the Society of Gynecologic Oncology (SGO) in January 2015 advised that using a HPV test by itself for cervical cancer screening offers an “effective alternative to the current recommendation for screening with either cytology (the Pap test) alone or co-testing with cytology and HPV testing,” according to ASCCP. They were specifically referring to the cobas HPV test, approved by the FDA in 2014 as a primary test for cervical cancer screening. For more information, see a related LabRoots article here.
The Practice Bulletin is gung-ho on vaccines to prevent HPV infection in females ages 9 to 26 years old, including the FDA-approved bivalent (Cervarix), quadrivalent (Gardasil), and most notably, the new 9-valent (Gardasil 9), which incorporates five additional high-risk HPV strains (versus the quadrivalent version). “Being able to prevent cervical cancer by vaccinating women against HPV is a remarkable achievement,” Chelmow says, noting the vaccines have proven repeatedly to be safe and effective. “ACOG (and I) wholeheartedly recommend vaccination. We have done a wonderful job at lowering the cervical cancer rate through routine screening, but vaccination can certainly take us an important step further. Countries like Australia who have implemented successful vaccination programs have already noted dramatic decreases in HPV-related diseases. It is essential that all health care providers (not just ob-gyns) discuss HPV vaccination with patients and with parents.” Chelmow is the Leo J. Dunn Distinguished Professor of Obstetrics and Gynecology, and chair, Department of Obstetrics and Gynecology, Virginia Commonwealth University School of Medicine, Richmond, Va.
Revised screening recommendations for women with HIV may have a significant effect on the quality of life and peace of mind for these patients. “Previous recommendations for women with HIV specified annual pap testing,” Chelmow notes. “Based on new data, co-testing is appropriate in women with HIV ages 30 and older, and women with negative co-testing can be screened every three years. This will be much more convenient for a substantial group of patients.”
Image Credit: David Chelmow, MD: Virginia Commonwealth University
Image Credit: ThinPrep Pap Smear: Clinical Microbiology Reviews, Eileen M. Burd Clin. Microbio. Rev. 2003;16;1-17. Copyright © American Society for Microbiology All Rights Reserved.
You May Also Like
Loading Comments...