FDA Approves First Sickle Cell Drug in 2 Decades
For the first time in almost two decades, patients with sickle cell disease will have access to a new drug thanks to a recent FDA approval.
The drug is known as Endari (L-glutamine oral powder), and it is approved for patients five years or older with sickle cell disease.
"Endari is the first treatment approved for patients with sickle cell disease in almost 20 years," said Richard Pazdur, M.D., acting director of the Office of Hematology and Oncology Products in the FDA's Center for Drug Evaluation and Research and director of the FDA's Oncology Center of Excellence. "Until now, only one other drug was approved for patients living with this serious, debilitating condition."
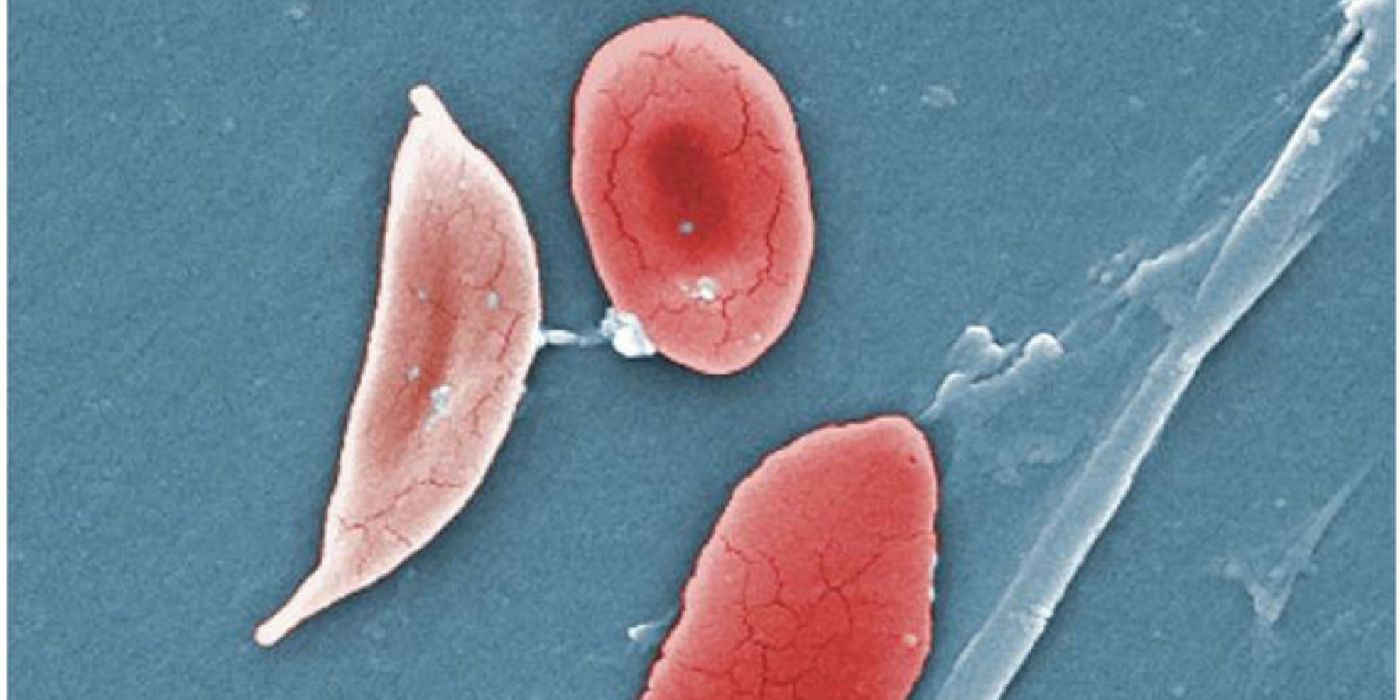
Sickle cell disease is a group of blood disorders characterized by abnormalities in the red blood cells. Under healthy circumstances, red blood cells resemble filled-in donuts that contain hemoglobin, a protein that carries oxygen. In sickle cell disease, the hemoglobin is defective, which causes the red blood cells to distort into a crescent, sickle shape. This malformed version is stiff and prone to clumping along the walls of blood vessels.
When sickled red blood cells block blood flow, tissues and organs become deprived of oxygen, which can cause major organ damage over time. Another complication of sickle cell disease is pulmonary hypertension - a high blood pressure condition caused by the blockages of the vessels in the lungs. Finally, sickled red blood cells don’t last very long in the body, and their premature breakdown contributes to anemia, a condition marked by low number of red blood cells. Sickle cell anemia is the most common type of sickle cell disease.
The FDA based its approval on randomized clinical trials that compared Endari to placebo in a group of patients with the disease. Over the course of 48 weeks, the research team showed that patients treated with Endari had less hospital visits for pain, and fewer days in the hospital. Endari-treated patients also had fewer instances of acute chest syndrome, a serious complication of sickle cell disease, as compared to placebo-treated patients.
With the approval, Endari could soon be accessible to the 100,000 Americans diagnosed with the disease. Of note, the inherited disease is more common in minority groups, such as Latinos and African Americans, in whom the disease affects 1 in every 13 people.
Although Endari showed compelling results in the clinical trial, it should not be mistaken for a cure. The drug was approved based on its capacity to "reduce severe complications associated with the blood disorder.” The drug also brings its own side effects, which include constipation, nausea, headache, cough, and chest pain. Nevertheless, Endari’s approval marks a great milestone for the hundreds of thousands of people living with sickle cell disease.