Drug for Huntington Disease Successfully Lowers Culprit Protein
In a study published in the New England Journal of Medicine, a drug for the first time was shown to successfully lower the abnormal protein that causes the debilitating Huntington disease (HD) in patients without any adverse reactions. The culprit protein is mutant huntingtin protein that becomes toxic in the central nervous system of Huntington disease patients.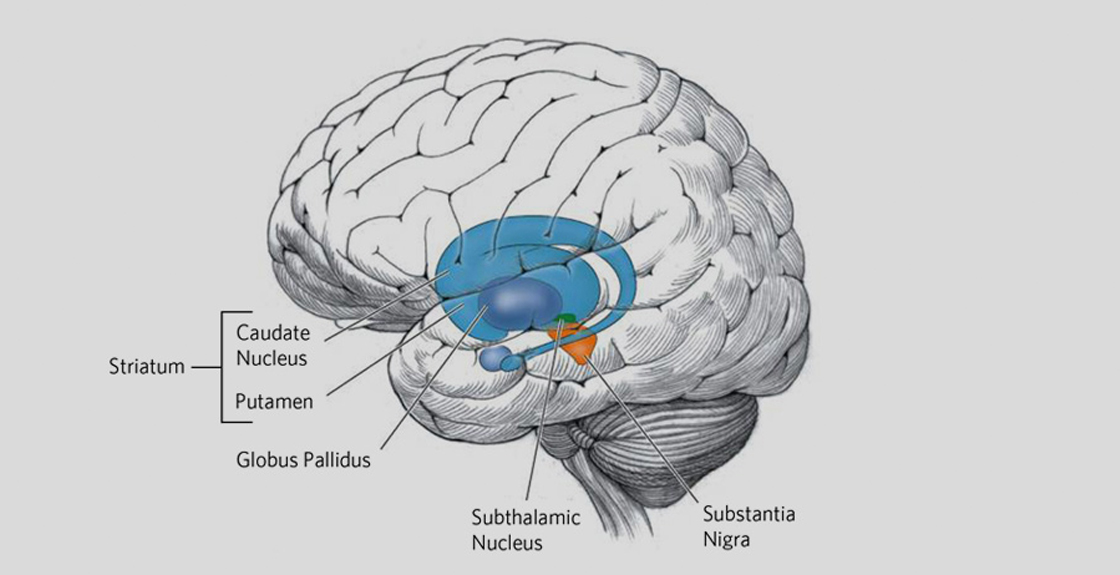
https://hdsa.org/wp-content/uploads/2019/03/what_is_hd_overview_hd_disease_full.jpg
“HD affects the whole brain, but certain areas are more vulnerable than others. Pictured above are the basal ganglia – a group of nerves cell clusters, called nuclei. These nuclei play a key role in movement and behavior control and are the parts of the brain most prominently affected in early HD.”
Credit: hdsa.org
The study is clinical trial designed to see if the drug halts or slows down progression of Huntington disease. The drug, sponsored by Ionis Pharmaceuticals and Hoffmann-La Roche, was administered monthly to 34 early HD patients via an injection directly into the cerebrospinal fluid (CSF)—which is the location of the protein.
"This is a tremendously exciting and promising result for patients and families affected by this devastating genetic brain disorder," said Dr. Blair Leavitt, neurologist and director of research at the Centre for Huntington Disease at UBC. "For the first time, we have evidence that a treatment can not only decrease levels of the toxic disease-causing protein in patients, but that it is also safe and very well tolerated."
Learn more about Huntington Disease (HD):
Huntington disease (HD) develops adulthood and progresses to causes abnormal involuntary movements, psychiatric symptoms and dementia. It is a fatal a genetic neurological disease caused by a single known mutation. There is currently no effective treatments that have been proven to slow the progression of the disease.
Source: Science Daily








