Inhibiting a Receptor Protein to Treat a Rare Form of Uterine Cancer
Uterine serous carcinoma is rare occurring in less than 10 percent of all cancer types of the endometrium. However, this particular uterine cancer is responsible for more than a third of the 10,000 endometrial cancer deaths per year.

This aggressive form of carcinoma doesn’t often lead to symptoms until the cancer has begun to move throughout the body. This leads to only about 8 months of progression-free survival with use standard chemotherapy and surgical treatments. These conventional treatments can slow the tumor growth. "The fact that these tumors grow rapidly, but also have a propensity to spread to lymph nodes and other organs very early, is a double whammy for women," explains associate professor of gynecology and obstetrics at the Johns Hopkins University School of Medicine, Amanda Fader, MD.
Fader was aware that about 30 percent of all uterine serous carcinomas tested positive for the receptor protein, HER2/neu, which is overexpressed in around 10 percent of all breast cancers. But, Trastuzumab (sold under the name of Herceptin), an oncological drug developed to treat certain types of breast cancer, can bind to and block HER2/neu signaling, allowing the tumor to stop growing.
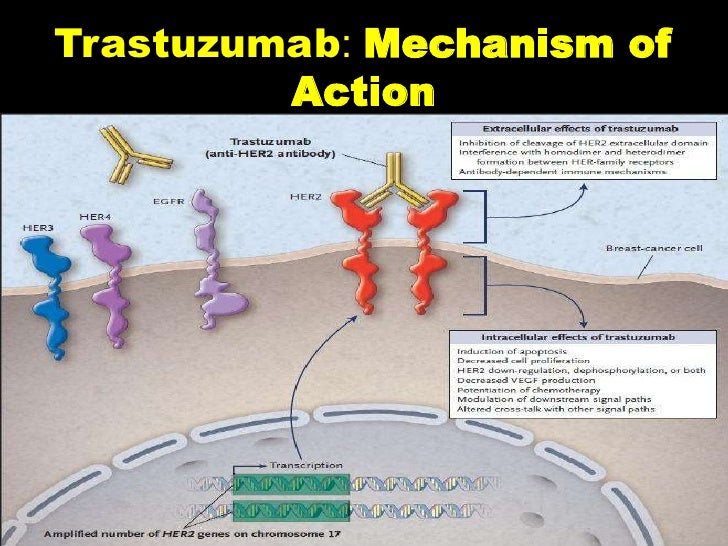
During a clinical study that lasted from August 2011 through March 2017, Fader and collaborators at other cancer treatment centers in the U.S. assigned 61 women randomly with uterine serous carcinoma to receive either the standard chemotherapy with a combination of carboplatin and paclitaxel — or trastuzumab in combination with carboplatin and paclitaxel.
A few of the 41 clinical study patients had advanced uterine serous carcinoma and about 17 had recurrent uterine serous carcinoma. All of these stages of carcinomas tested positive for the HER2/neu receptor. The 28 patients’ receiving the control, which is the standard carboplatin and paclitaxel, had an average of 8 months average progression-free survival. However, the 30 patients that received the trastuzumab in addition to the combination drugs had a progression-free survival time of 12.6 months. Perhaps the most interesting part of trastuzumab in cocmabaition of other drugs was that in the 41 patients with advanced uterine serous carcinoma, the progression-free survival time went from an average of 9.3 months to 17.9 months. "Even an improvement of a few months may be quite meaningful for women with these cancers," explains Fader.
Furthermore, patients with recurrent disease, progression-free survival time increased from just six months to 9.2 months. The only difference was that these patients have been extensively treated before, and thus are more likely to have poor health, mutated tumors, and tumors with different levels of HER2. Fader believes that using trastuzumab to selectively treat uterine serous carcinoma that express HER2/neu is part of precision medicine.
Sources: Journal of Clinical Oncology, Medical Xpress








