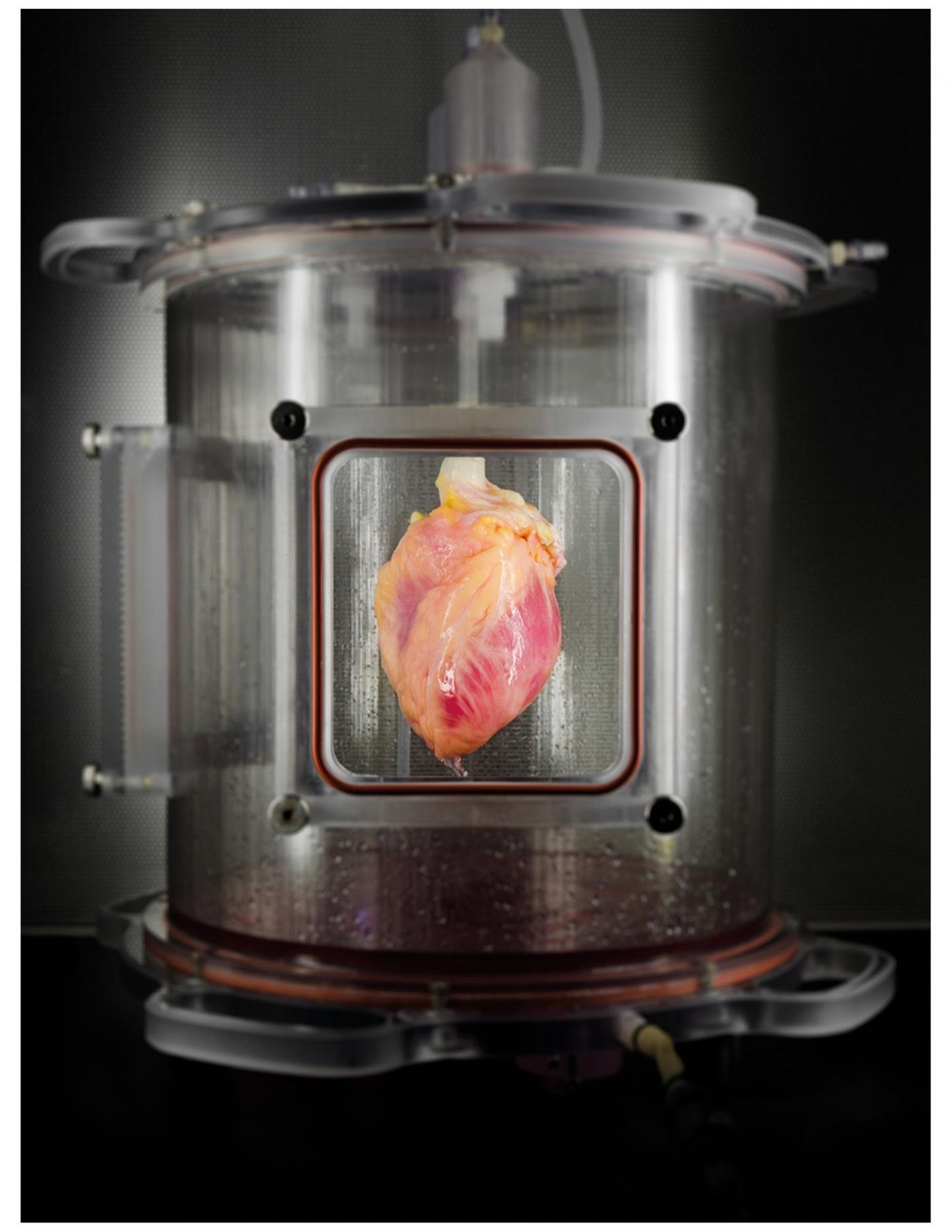
Researchers have developed functional heart tissue in a lab. Their work can be used to help grow heart tissue patches using the patient’s own cells.
The Massachusetts General Hospital (MGH) scientists created the tissue using donor hearts stripped of components that would generate an immune response. They additionally used cardiac muscle cells generated from induced pluripotent stem cells. Pluripotent stem cells are cells taken from any tissue (usually the skin or blood) of a person and reengineered to behave like an embryonic stem cell. In this case, the pluripotent stem cells could be taken from a potential recipient.
"Generating functional cardiac tissue involves meeting several challenges," said biomedical engineer Jacques Guyette, the lead author of the paper. "These include providing a structural scaffold that is able to support cardiac function, a supply of specialized cardiac cells, and a supportive environment in which cells can repopulate the scaffold to form mature tissue capable of handling complex cardiac functions.”
The researchers grew a scaffold from proteins secreted by the cell to give the tissues shape. They used 73 donor hearts, deemed unfit for transplant, to create the tissue. Using a method invented by team leader Harald Ott in 2008, the scientists stripped living cells out of the donor organs (decellularization). They then repopulated the remaining extracellular matrix scaffold with organ-appropriate cell-types (recellularization). In this recellularization step, messenger RNA was used to regenerate skin cells back to stem cells. The resulting pluripotent stem cells were then programmed to grow cardiac muscle cells.
The organs were then mounted for 14 days in an automated bioreactor system the researchers previously developed. The automated bioreactor system has the ability to support a whole human heart during the recellularization process.
The researchers’ method led to a high retention of matrix proteins and a structure without cardiac cells. The method also allowed for the preservation of coronary vascular and microvascular structures.
Creating a bioengineered human heart is "most certainly a long-term goal that is several years away," says Guyette. "We are currently working on engineering a functional myocardial patch that could replace cardiac tissue damaged due a heart attack or heart failure."
"Among the next steps [include] improving methods to generate even more cardiac cells - recellularizing a whole heart would take tens of billions, optimizing bioreactor-based culture techniques to improve the maturation and function of engineered cardiac tissue, and electronically integrating regenerated tissue to function within the recipient's heart."
Ott adds that "generating personalized functional myocardium from patient-derived cells is an important step towards novel device-engineering strategies and will potentially enable patient-specific disease modeling and therapeutic discovery. Our team is excited to further develop both of these strategies in future projects."
The research was published on March 11, 2016, in the journal
Circulation Research.
Sources:
Massechussetts General Hospital press release via EurekAlert!,
Circulation Research
 Researchers have developed functional heart tissue in a lab. Their work can be used to help grow heart tissue patches using the patient’s own cells.
Researchers have developed functional heart tissue in a lab. Their work can be used to help grow heart tissue patches using the patient’s own cells.