Convalescent Plasma Therapy 'Keeps up' With COVID Variants
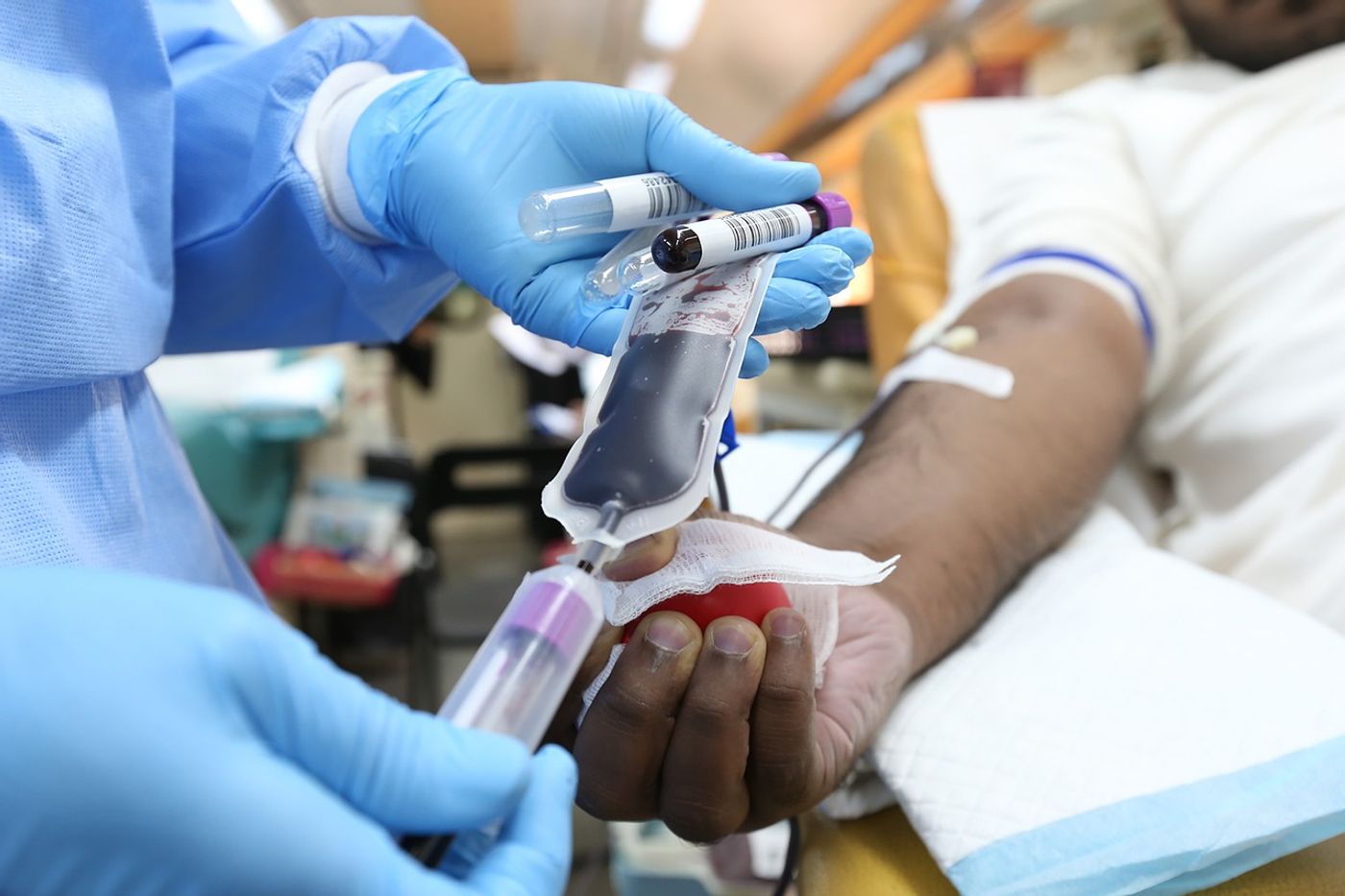
A recent study has demonstrated that COVID-19 convalescent plasma treatment (or blood from individuals who have recovered from the disease) can slash the risk of hospitalization by over 50 percent. These plasma therapies are rich in antibodies against SARS-CoV-2, produced by the immune systems of patients who have successfully fought off the virus.
As the pandemic rages on worldwide, low- and middle-income countries are in desperate need of accessible frontline COVID-19 therapies to save lives. In these settings, vaccines, monoclonal antibodies, and other COVID therapeutics may not be readily available, which forced researchers from the Johns Hopkins University School of Medicine to seek out potential alternatives.
David Sullivan led a study involving over 1000 randomized adult COVID-19 patients who received one of two transfusion treatments for their infection: convalescent plasma or a control plasma (without SARS-CoV-2 antibodies). All the patients in the study had tested positive for COVID in the week leading up to receiving the transfusion.
Sullivan and colleagues defined treatment success as a patient who did not require hospitalization for their symptoms within around a month of receiving the transfusion. The researchers reported that 2.9 percent of those who received convalescent plasma needed to be admitted into the hospital. Conversely, 6.3 percent of those in the placebo group were hospitalized, which translates to a risk reduction of 54 percent with the convalescent plasma.
Sullivan says that this represents a viable option to help reduce hospitalizations, adding that convalescent plasma therapy is the only one that “keeps up with SARS-CoV-2 variants,” including the Delta and Omicron strains currently circulating globally because the plasma contains neutralizing antibodies specific to those variants.
In the United States, convalescent plasma therapy has been given an emergency use authorization designation by the Food and Drug Administration, limiting its use for COVID-19 outpatients.
“We have shared our findings with the FDA, as well as with the World Health Organization,” said Sullivan. “We hope that both organizations will see the value of convalescent plasma for outpatients based on the strength of our study, the largest randomized clinical trial of its kind to date.”
Despite the success of this trial, data from previous studies showed that convalescent therapy did not improve outcomes for patients at the early stages of COVID-19 infection. In this prior study, the authors attributed the failure of plasma therapy to several factors, including insufficient plasma doses, the timing of plasma administration, host-related factors, or other aspects of the host tissue responses to the infection.