Errors During B Cell Metabolism Cause Lymphoma and Other Diseases
B cells are the multi-taskers of the immune system, and their different duties require different amounts of energy. A new study focused on the protein that acts as a B cell “handler,” and it could be a target for future treatments for different diseases, including lymphoma.
Depending on whether B cells are actively involved in an immune response, resting dormant or busy cultivating immune memory, they have different levels of metabolic needs to keep them functioning. A protein called GSK3 controls B cell metabolism to meet the needs of B cells, whether they are in a germinal center or in circulation.
"Our research shows that the protein GSK3 plays a crucial role in helping B cells meet the energy needs of their distinct states," said Robert Rickert, Ph.D. "It acts as a metabolic sensor, or checkpoint, that promotes the survival of circulating B cells while limiting growth and proliferation of B cells in germinal centers.”
For circulating cells, GSK3 prevents B cells from going overboard on metabolic activity. But for cells in a germinal center, where B cells proliferate and “hypermutate” their antibody genes to produce the most high-affinity antibodies, GSK3 limits glycolysis and mitochondrial production to ensure germinal B cell survival.
“The findings are particularly relevant for certain B cell pathologies, including lymphoma subtypes, where there is an increased demand for energy to support the hyperproliferation of cells in a microenvironment that may be limited in nutrients,” Rickert said.
Rickert’s study focused on why GSK3 is so vital to germinal B cells, which require large amounts of energy. To obtain the energy they need, they consume a lot of sugars, fatty acids, and amino acids to the point where they consume nearly all of the glucose that’s available to them. Then they result to obtaining energy from glycolysis, a secondary, less efficient, and non-oxygen-dependent energy production method.
Unfortunately, too much glycolytic activity leads to an overload of reactive oxygen species (ROS), which is toxic to the body’s cells. Therefore, GSK3 is saving germinal B cells from ROS-induced cell death because of its ability to limit glycolysis.
"Until now, we would have thought that slowing metabolism would only be important for preventing B cells from becoming cancerous, which it indeed may be,” Rickert said. “These studies provide insight into the dynamic nature of B cell metabolism that literally 'fuels' differentiation in the germinal center to produce an effective antibody response."
The recent study could support new studies for treating lymphoma, depending on how this particular type of cancer is related to B cell metabolism. "It's not yet clear whether or how GSK3 might be a target for future therapies for B cell-related diseases,” Rickert explained. “But this research opens a lot of doors for further studies.”
Rickert’s research was recently published in the journal Nature Immunology.
Source: Sanford-Burnham Prebys Medical Discovery Institute
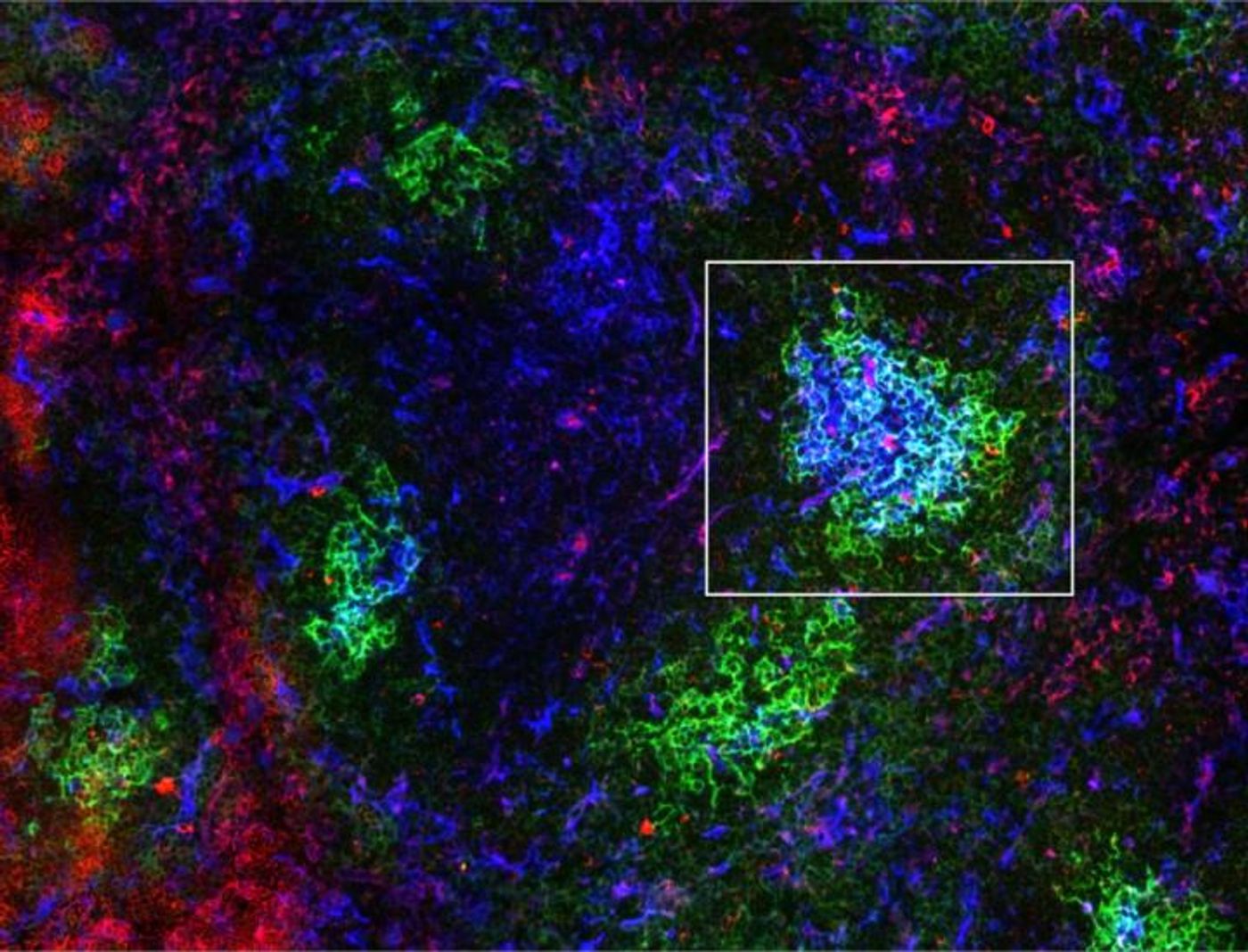
Image credit: Laboratory of Robert Rickert, Ph.D., at Sanford Burnham Prebys Medical Discovery Institute