Sex Chromosomes to Blame for Women's Risk of Autoimmune Diseases
Why are women more likely to have an autoimmune disease? It may literally be in their genes. A new study suggests that when a process to balance out genes from the “extra” X chromosome found in females goes awry, dysfunction in the immune system can lead to autoreactive diseases.
The genetic difference between males and females, as most people know, is that males have two different sex chromosomes (X, Y) and females have two of the same chromosome (X, X). However, females don’t need genes on both X chromosomes to be expressed, so a random process called X chromosome inactivation results in one X chromosome being invisible, often called a barr body. If the process is successful, no genes from the barr body are expressed. Now, researchers have reason to believe that some genes on the barr body, the “inactive X,” in immune cells are being expressed. And with extra immune genes being activated, autoimmune responses can develop.
From the University of Pennsylvania, scientists looked at the repercussions of an “extra dose” of “immunity-related gene expression” from the inactive X.
They build upon past findings that showed how the X chromosome inactivation process in female B and T cells often failed to reach completion. This was due to a long non-coding RNA (lncRNA) called Xist that failed to “initiate and maintain” the process. However, this failure was only before the immune cells were stimulated in response to an infection. The priming that takes place to prepare immune cells for the immune response, for whatever reason, caused Xist to (finally) show up in the right place.
“B cells are the ones making antibodies and autoantibodies, so they're really crucial in both protective immune responses and autoimmunity," described senior author Montserrat C. Anguera. "A big question that remains is, why are these immune cells priming for this chromosome to be regulated differently and also, if these processes go awry, how does that lead to autoimmunity and loss of self-tolerance?"
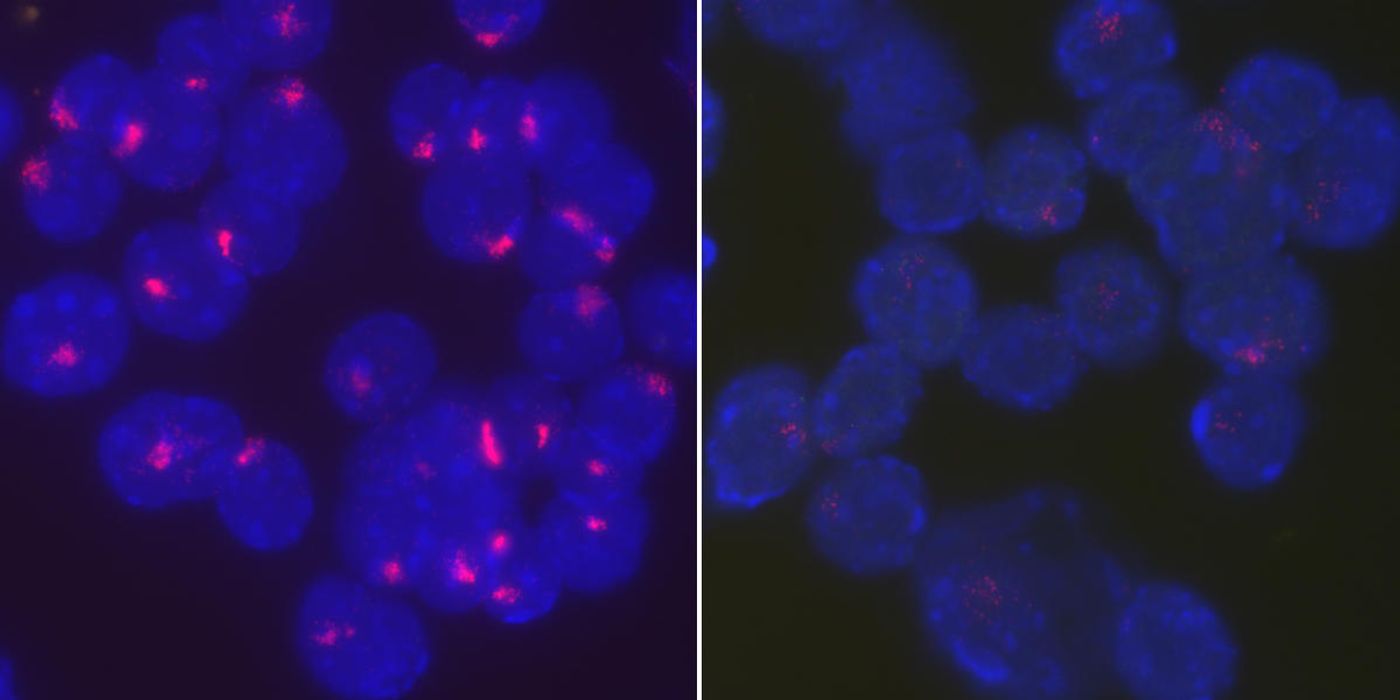
To answer these questions, the present study began to identify the factor that brings Xist RNA back during immune cell activation. Researchers actually tracked Xist during B cell development in female mice. At the beginning of development, the cells that come before full-fledged B cells - hematopoietic stem cells, common lymphoid precursors - at first seemed to show “clear patterns of Xist RNA on the inactive X chromosome.” But at some point Xist disappeared and reappeared, but when it came back, it was not in the place it needed to be to initiate X chromosome inactivation.
While tracking Xist, they also saw that an absence of heterochromatin modifications, “small-molecule tags” associated with gene repression during X chromosome inactivation.
Xist gathered in the inactive X during B cell activation, finally entering into a position capable of beginning X chromosome inactivation. Researchers pinned down the return of Xist to two different phases:
-
Between four and 16 hours after B cell stimulation they saw “speckles” of Xist
-
Between 16 and 30 hours after stimulation, they saw Xist “concentrated exclusively” at the inactive X
In addition, heterochromatin modifications increased and gathered in the second phase too.
During the study, researchers also realized how important it was for transcription factor YY1 to be involved. In mice B cells lacking YY1, researchers observed “greatly reduced levels of heterochromatin marks” and reduced “localization of Xist RNA to the inactive X.” This result signified to the researchers how the DNA binding activity of YY1 must be responsible for transporting Xist RNA back to the inactive X.
"It seems to be acting as a tether, bringing the Xist RNA together with the DNA of the inactive X chromosome," Anguera explained. "If you want to develop a therapy for autoimmune diseases, the idea is, how do we get Xist to the inactive X chromosome and keep it there so we maintain dosage compensation in these B cells. Certainly YY1 is looking like a really promising target."
The present study was published in the journal PLOS Genetics.
Learn more about the process of X chromosome inactivation:
Source: University of Pennsylvania