According to the American Cancer Society, more than 221,000 Americans will be diagnosed with lung cancer in 2015. Positive non-small cell lung cancer (NSCLC) accounts for 85 percent of all lung cancers. Approximately 60 percent of lung cancer diagnoses in the United States are made when the disease is in the advanced stages.
Genentech, a member of the Roche Group, has received a second Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA) for its investigational cancer immunotherapy MPDL3280A (anti-PDL1). The designation was granted for the treatment of people with PD-L1 (Programmed Death-Ligand 1) NSCLC whose disease has progressed during or after platinum-based chemotherapy (and an appropriate targeted therapy for those with an EGFR mutation-positive or ALK-positive tumor). These checkpoint blockade immunotherapies work by reawakening the immune system's response to tumors.
"Lung cancer is the leading cause of cancer death globally," said Sandra Horning, M.D., chief medical officer and head of Global Product Development. "We are committed to personalized healthcare, developing medicines like MPDL3280A with companion tests that may help us identify those who may be appropriate candidates for our medicines."
Genentech currently has two approved medicines to treat certain kinds of lung cancer and more than 10 medicines being developed to target the most common genetic drivers of lung cancer or to boost the immune system to combat the disease. The company is investing in an effort to offer treatment options that help a person's own immune system fight cancer. Its personalized cancer immunotherapy research and development program comprises more than 20 investigational candidates, seven of which are in clinical trials.
The Breakthrough Therapy Designation is based on early results of MPDL3280A in people whose NSCLC was characterized as PD-L1 positive by an investigational test being developed by Roche. All studies of MPDL3280A are prospectively evaluating PD-L1 expression. Some studies will evaluate the medicine regardless of a tumor's PD-L1 status, while other studies are evaluating the medicine only in people whose tumors are characterized as PD-L1 positive.
Breakthrough Therapy Designation is designed to expedite the development and review of medicines intended to treat serious diseases and to help ensure that patients have access to them through FDA approval as soon as possible. The FDA granted the first Breakthrough Therapy Designation for MPDL3280A in metastatic bladder cancer in 2014. Ongoing pivotal studies of MPDL3280A include lung and bladder cancer, and Genentech plans to initiate Phase III studies in additional tumor types this year.
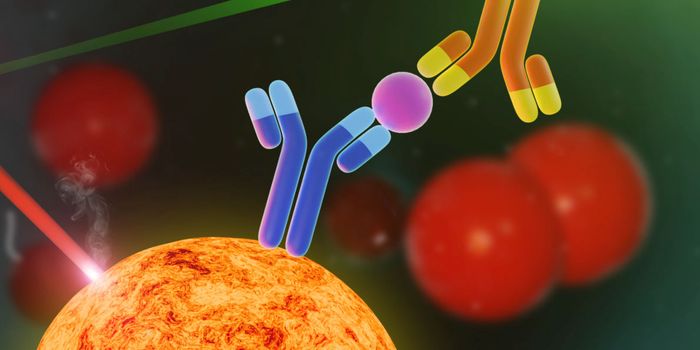
The investigational monoclonal antibody is designed to interfere with a protein called PD-L1. It is designed to target PD-L1 expressed on tumor cells and tumor-infiltrating immune cells, preventing it from binding to PD-1 and B7.1 on the surface of T cells. By inhibiting PD-L1, MPDL3280A may enable the activation of T cells, restoring their ability to effectively detect and attack tumor cells.
 According to the American Cancer Society, more than 221,000 Americans will be diagnosed with lung cancer in 2015. Positive non-small cell lung cancer (NSCLC) accounts for 85 percent of all lung cancers. Approximately 60 percent of lung cancer diagnoses in the United States are made when the disease is in the advanced stages.
According to the American Cancer Society, more than 221,000 Americans will be diagnosed with lung cancer in 2015. Positive non-small cell lung cancer (NSCLC) accounts for 85 percent of all lung cancers. Approximately 60 percent of lung cancer diagnoses in the United States are made when the disease is in the advanced stages.