Researchers Develop an Experimental Therapy for Triple-negative Breast Cancer
Between twelve and seventeen percent of all breast cancer cases are triple-negative, a highly aggressive type. It lacks three common cancer therapeutic targets: human epidermal growth factor receptor 2 (HER2) and the progesterone (PR) and estrogen receptors (ER). Now researchers have created an experimental therapy for this deadly cancer, which was able to slow the metastasis of triple-negative breast cancer in a mouse model. The therapeutic protein, Tinagl1, can block two main pathways that breast cancer cells use to grow and metastasize, and is based on a natural protein. The findings were reported in Cancer Cell.
"People have tried to block the spread of this form of cancer, but attempts so far have failed because if you try one approach, the cancer cells compensate by finding a way to escape," noted the senior author of the work Yibin Kang, the Warner-Lambert/Parke-Davis Professor of Molecular Biology at Princeton University and associate director for consortium research at the Rutgers Cancer Institute of New Jersey. "With this new approach, the treatment blocks both pathways at the same time. It is like having one stone that kills two birds."
There aren't many treatment options for patients with triple-negative breast cancer; there is a high chance of relapse, and the prognosis is poor. This cancer can also become resistant to treatment. This work shows that there may be an option for these individuals one day.
The scientists determined that Tinagl1 was able to stop the action of a protein called epidermal growth factor receptor gene (EGFR), which can promote tumor growth; if the gene that encodes for EGFR contains mutations, its activity increases, which fuels the growth of tumors and encourages cells to break off and migrate to other parts of the body. Therapeutics that target EGFR have not been particularly effective, potentially because cancer cells can use alternative pathways when EGFR is down. Tinagl1 was able to inhibit EGFR.
The other pathway that was impacted by the therapeutic involves molecules called integrins, which help control cell movement and adhesion, and the shift to malignancy. Tinagl1 seems to disrupt integrin signaling by acting on another protein, focal adhesion kinase (FAK), which also functions in cell growth and migration as well as survival. These pathways are physiologically connected, with redundancy and compensation that enables cancer to evade treatment and grow aggressively, noted the scientists. The team found that both were inhibited by the experimental therapeutic.
Over 800 samples of human breast tumors were assessed for this work, which showed that tumor samples with lower Tinagl1 gene expression levels tended to come from advanced cancers and patients with shorter survival times. When tumors had high Tinagl1 expression levels, patients were more likely to have a good prognosis. In the triple-negative breast cancer samples, those differences were most pronounced.
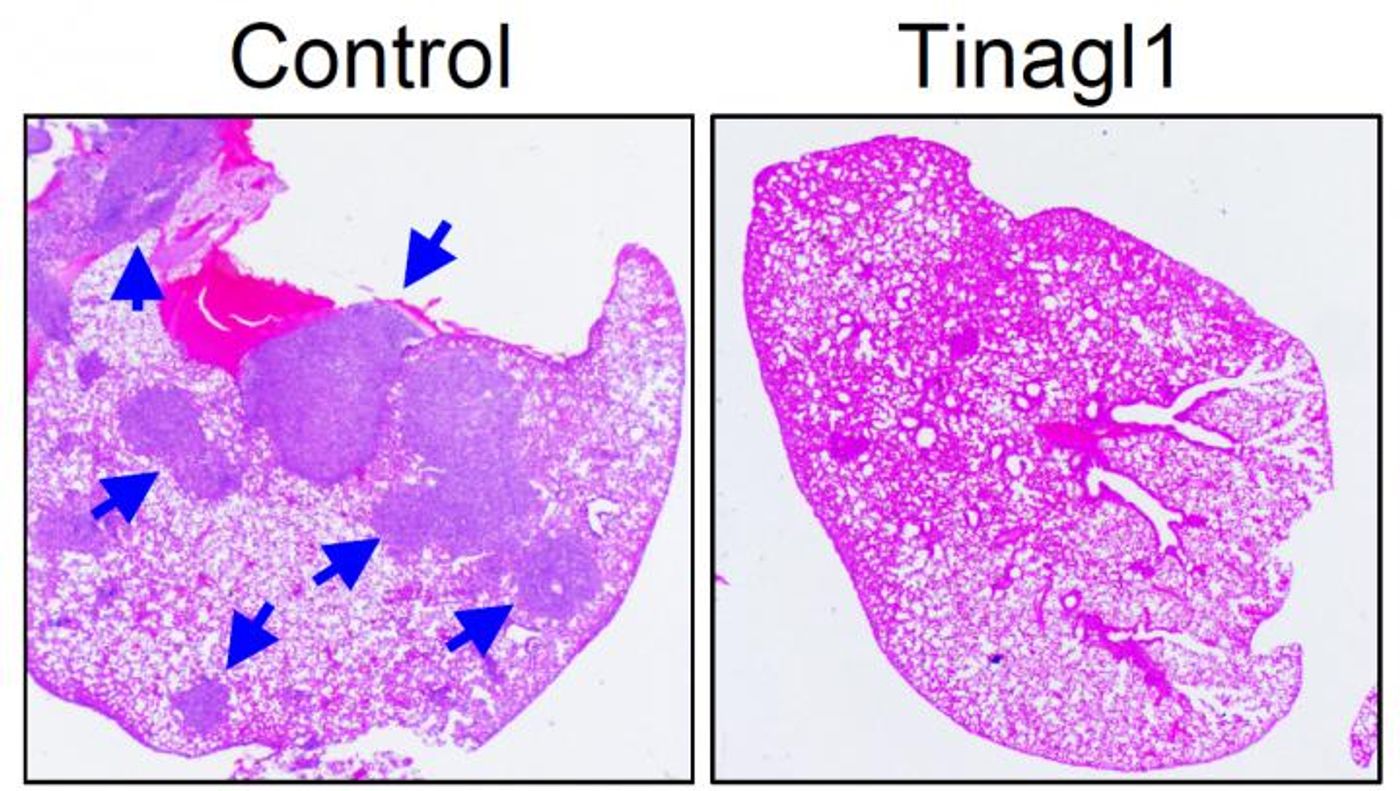
After exposing mice with mammary tumors to the Tinagl1 protein for seven weeks, the researchers found that the growth of the primary tumors in the mice was inhibited, and there was less metastasis to the lungs. That treatment had an impact even when the metastasis had already started.
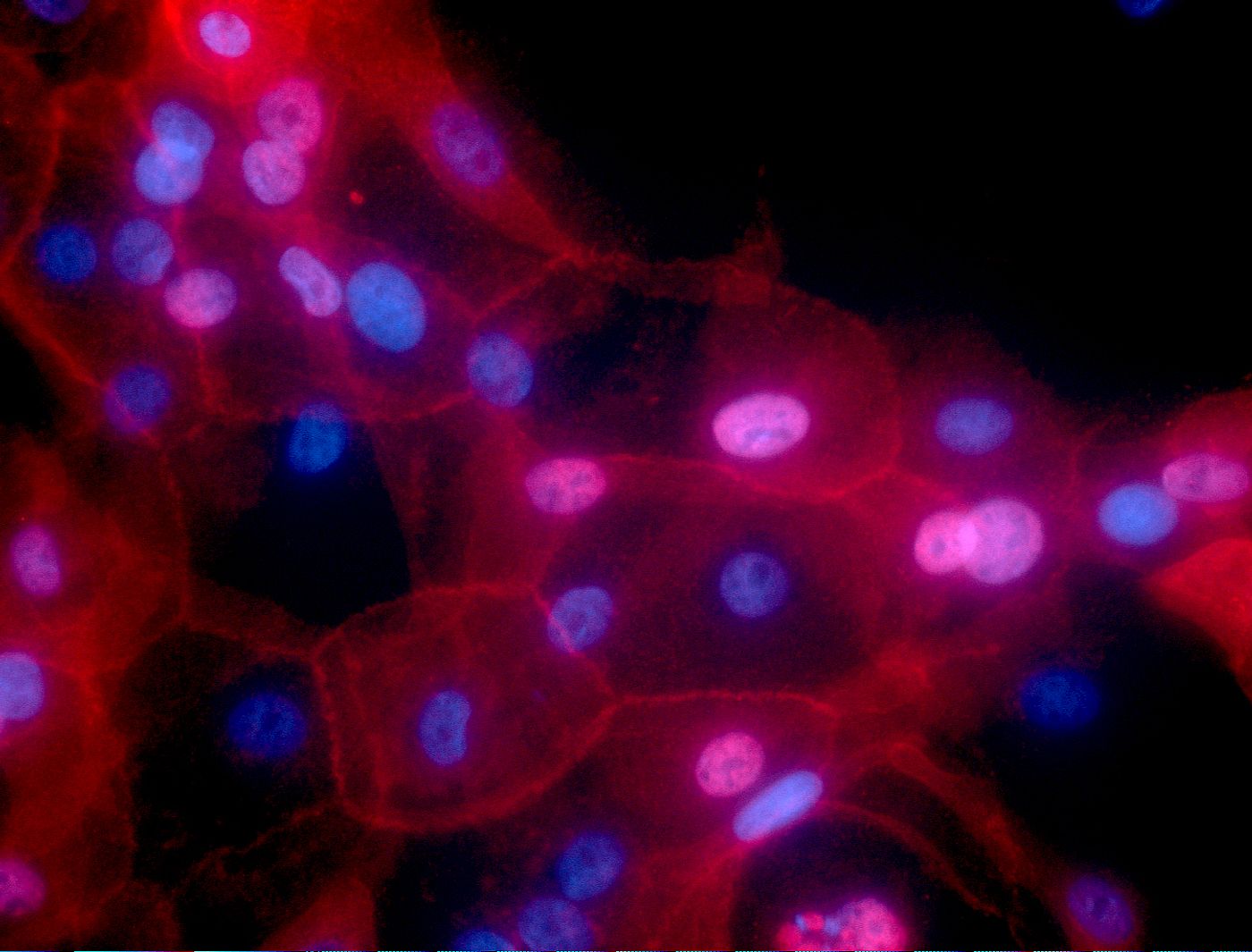
The researchers also investigated whether the Tinagl1 gene helps shield against the growth and spread of tumors by engineering tumor cells with high Tinagl1 gene expression. In mouse cancer cells, high expression levels reduced cancer growth and metastasis. However, mouse models have been cured of cancer before. This research does not necessarily mean this therapy will be effective in humans. Much more follow-up work will be needed.
In the video above, Kang discusses breast cancer and metastasis.
Sources: AAAS/Eurekalert! via Princeton University, Cancer Cell