Imagine a nanoscale needle that can pierce cells precisely and on command. Scientists from Harvard’s Wyss Institute for Biologically Inspired Engineering and Harvard Medical School (HMS) have developed just this, possibly revolutionizing the future of drug delivery.
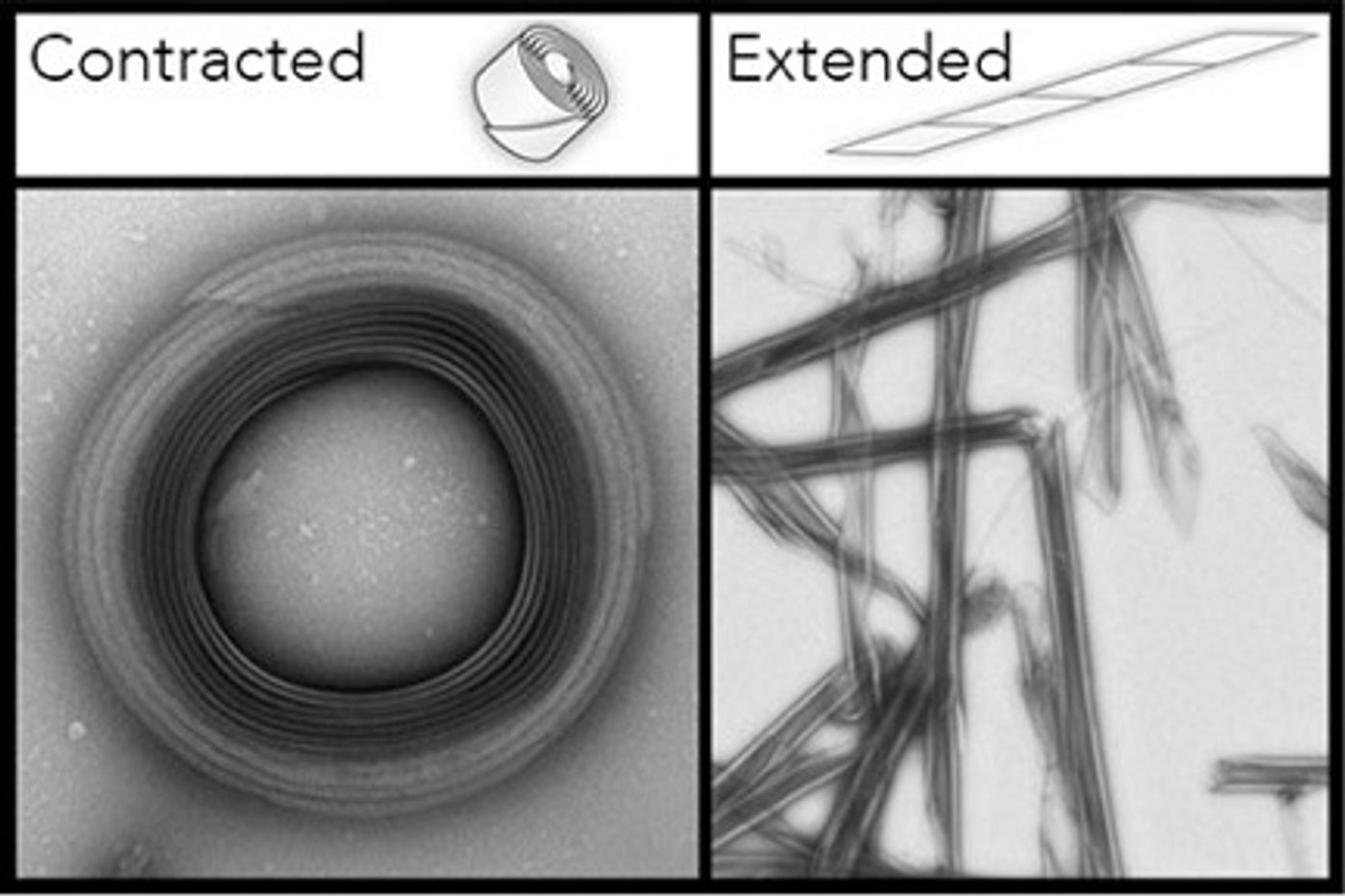
Published in the American Chemical Society Synthetic Biology, the discovery adapts bacterial protein polymers known as “R bodies” into precise, retractable nanoneedles. Controlled by pH levels, the nanoneedles extend to puncture cell membranes to release encapsulated cargo. Once pH levels change, they quickly reverse to the coiled form, ready for action yet again.
"This is one of nature’s innovations, but the discovery here is our ability to translate this from nature into a system that we can now engineer and control," said Pamela Silver, senior author on the study and Wyss Core Faculty member.
In nature, R bodies are produced by the
Paramecium endosymbionts as a form of deadly self-defense. R bodies exist as coiled proteinaceous ribbons at low pH. In response to being ingested by another bacterial strain, the “killer strain” uses the differences in pH levels to activate its killer trait – extension of the R bodies – to rupture the host cell’s membrane. Toxins are then released which kills the host paramecium.
Impressed by these biological javelins, the research team at Harvard adapted this defense mechanism into a new way to deliver drugs to targeted cells. They expressed and purified the R bodies from
E. coli. They then fine-tuned the extension process by screening for mutants that have altered pH sensitivity. Ultimately, they derived at a system of synthetic pistons that respond to pH levels to puncture
E. coli membranes, releasing encapsulated cargo.
"Our research establishes R bodies as biological machines that we can use to break through membranes,” said Jessica Polka, first author of the study. “These actuators don’t consume molecular fuel and are extremely robust; we believe they could one day be used to deliver material to mammalian cells."
The system has many advantages, including its tunable platform, which allows for controlled release of trapped content. The authors see this as a potential way to deliver toxins or other pharmaceutical payloads directly to targeted cells. For cancer, this system could more effectively kill tumor cells and reduce collateral damages to healthy cells.
Another property that makes this delivery system quite unique is its reversibility. Because the extension and retraction actions are purely mechanical, they can be controlled with great precision and can be re-used over and over again. This is highly beneficial considering the many compartments that are contained in eukaryotic cells.
The sky seems to be the limit for these new biological actuators. “By decorating these polymers with different kinds of metals or other materials with properties of interest, we could potentially develop a range of actuator-like, nanoscale structures," said Silver.
"There are so many amazing mechanisms engineered by Nature, and this is a great example of how we can mine living systems for unique biological elements and adapt them to develop programmable technologies for high value applications using synthetic biology," said Donald Ingber, Wyss Institute Founding Director.
Additional source:
Wyss Institute