The U.S. Food and Drug Administration just approved a new line of treatment for obese patients who struggle with the conventional non-surgerical weight-loss therapy. The newly approved device, named AspireAssist, involves a tube connected to the stomach that will drain a small amount of digested food after every meal. In effect, the device resembles a carefully controlled stomach pump, which has incited fierce criticisms amongst health experts.
"The AspireAssist approach helps provide effective control of calorie absorption, which is a key principle of weight management therapy," said William Maisel, deputy director for science and chief scientist in the FDA's Center for Devices and Radiological Health.
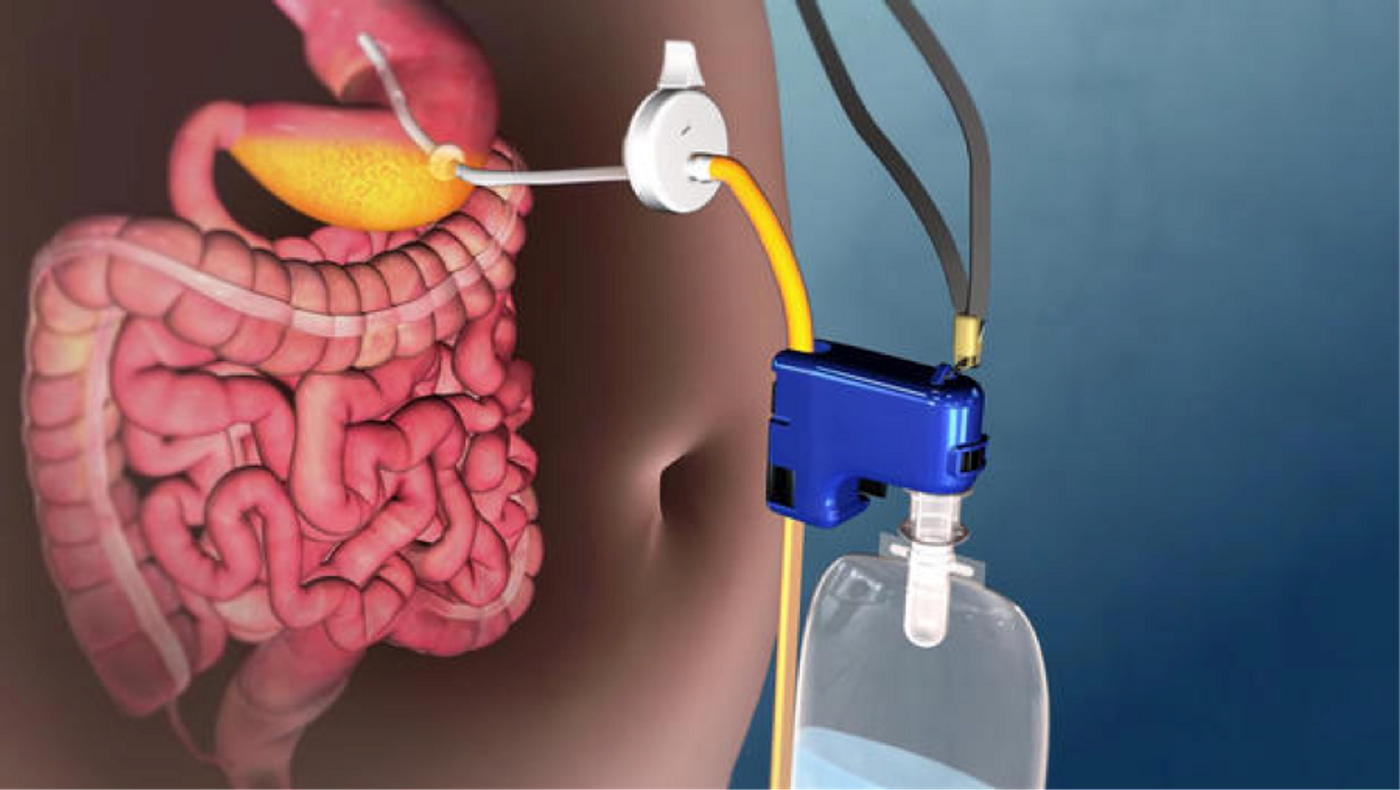
Indeed, the AspireAssist’s success is through preventing the body from absorbing 100 percent of the foods the patient ingests. It starts with the surgeon placing a tube in the stomach. Connected to this tube is a port valve that’s flushed against the abdomen, on the outside of the body. Then after every meal, the patient drains out part of the stomach’s contents via an external connector to the port valve.
In clinical trials, the device demonstrated a 30 percent reduction in the calories absorbed by patients. Furthermore, of the 111 patients treated with AspireAssist, the average weight loss was 12.1 percent of their total body weight. This is significantly higher than the 3.6 percent lost in the control group who only received lifestyle therapy.
To help patients stay on track and prevent abuse of the device, makers have put safety mechanisms in place. For example, the device is programmed to stop working after 115 cycles, which equates to about 5 to 6 weeks of therapy. This means that doctors are closely monitoring patients to ensure proper use. In addition, this safety measure makes sure that the device is checked frequently for infections and other complications.
Nevertheless, it’s not enough to just be outfitted with the AspireAssist device. For patients to achieve healthy weight loss and maintain their progress, physicians agree that counseling and behavioral therapies are not optional. "Patients need to be regularly monitored by their health care provider and should follow a lifestyle program to help them develop healthier eating habits and reduce their calorie intake," said Maisel.
Not everyone who desires weight loss will be eligible for the AspireAssist. The device is only intended to help those who are over 22 years old, and are clinically obese, as defined by having a body mass index of 35 to 55. They must also have demonstrated history of failed weight loss via conventional methods that didn’t involve surgery.
Of course, as with any surgical procedure, the AspireAssist is not without risks. But for patients who need this help, doctors and the FDA agree that the benefits of weight loss exceed the risks of the procedure. However, critics of the device have gone as far as calling this a “
bulimia machine” and wondered whether an at-home stomach pump is actually going a step too far.
Additional source:
FDA release