'Acetazolamide' Used To Treat Altitude Sickness, Slows Down the Development of Glioblastoma
Acetazolamide, which is sold under the trade name Diamox and used to treat altitude sickness, is considered inexpensive to manufacture and easy to administer.
"I take it myself, whenever I go to the Rocky Mountains," explains study director Bahktiar Yamini, MD, a professor of neurosurgery at the University of Chicago Medicine. Although acetazolamide has minimal side effects, users report "a metallic taste when drinking something carbonated."
Acetazolamide is a studied to be a carbonic anhydrase inhibitor drug that can restore another drug's ability to eradicate tumor cells.
More specifically, acetazolamide when added to a drug called temozolomide (TMZ), it was seen to slow down the progression of glioblastoma in mouse models.
Acetazolamide enables TMZ to damage the DNA present in tumor cells in order to inhibit its growth.
Researchers discovered that glioblastoma patients with increased levels of a protein called BCL-3 (B cell CLL/lymphoma 3) were unresponsive to the effects exerted by the chemotherapeutic drug, TMZ. In particular, BCL-3 protects cancer cells from TMZ damage by activating a protective enzyme known as carbonic anhydrase II.
"We tested this combination treatment strategy in several animal models," Yamini said. “It cured some of them. Others had a 30 to 40 percent increase in survival time.”
Furthermore, the researchers examined BCL-3 levels from earlier human studies and found that patients with lower levels of BCL-3 who were treated with TMZ had a higher survival rate than patients who had high levels of this biomarker.
"An important feature of predictors like BCL-3 is that they are informative," the authors note. "They can identify pathways to improve treatment response."
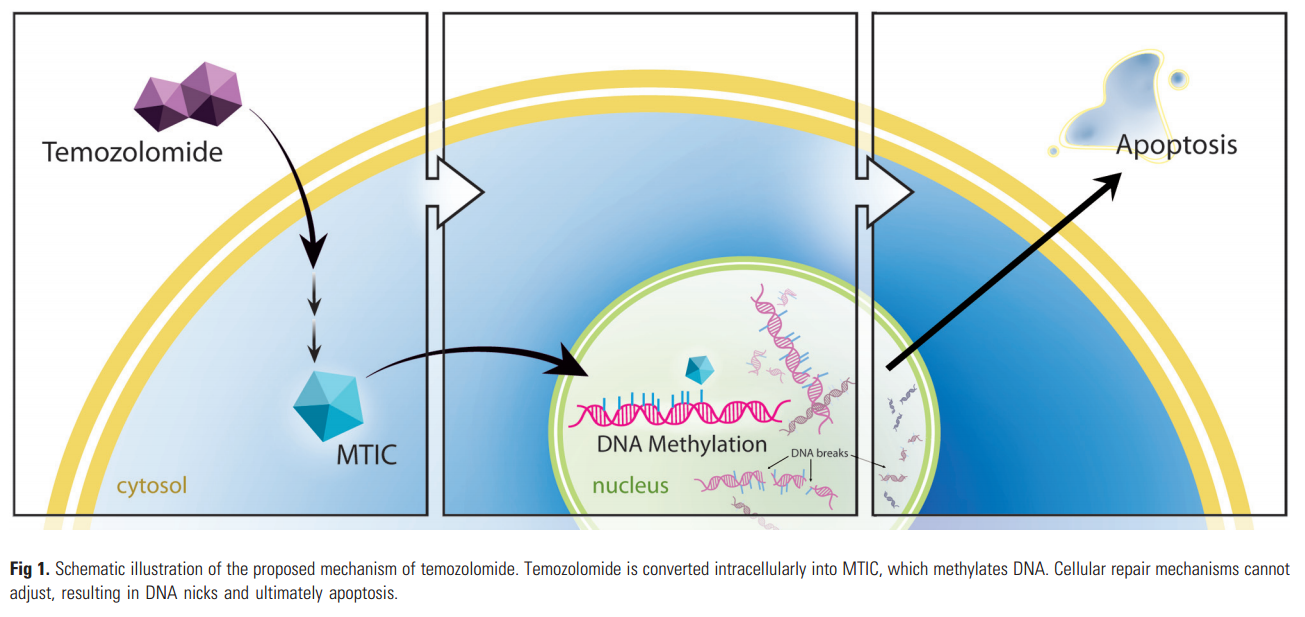
By studying those pathways, carbonic anhydrase inhibitors were identified, such as acetazolamide, as a way to decrease the resistance to temozolomide.
"Our data," they note, demonstrate that it is the "induction of CAII by TMZ that is important in modulating response to therapy."
Confirming the use of BCL-3 to predict which patients will benefit from the use of temozolomide will require validation through clinical trials.
Additionally, the authors believe that repurposing the use of acetazolamide along with temozolamide might be particularly effective for patients with tumors that have high BCL-3 expression.








