New Two-Drug Combination in Treating Hepatitis C
A new drug by the name of ‘ravidasvir’ is part of a hepatitis c combination treatment that may be an affordable option for patients. Ravidasvir was seen to meet safety standards and efficacy with high cure rates in affected individuals, including the hard-to-treat cases. "The results indicate that the sofosbuvir/ravidasvir combination is comparable to the very best hepatitis C therapies available today but it is priced affordably and could allow an alternative option in countries excluded from pharmaceutical company access programs," explains Bernard Pécoul Executive Director, DND.
The clinical trial examined medicines manufactured by Egyptian drug manufacturer Pharco Pharmaceuticals and co-sponsored by the Malaysian Health Ministry. "As hepatitis C has become a major public health concern in Malaysia, it is crucial to increase access to treatment for the benefit of the nation," notes Datuk Dr Noor Hisham Abdullah Director General of Health, Ministry of Health, Malaysia. In fact, the government of Malaysia released a "government-use" license on sofosbuvir patents to allow around 400,000 Malaysians living with hepatitis C to receive basic HCV regimens in public hospitals.

DND administrated the STORM-C-1 open-label trial in order to measure the efficacy, safety, tolerance, and pharmacokinetics of the drug ravidasvir in combination with sofosbuvir. Chronically infected adults without cirrhosis of the liver were treated with the drug combination for 12 weeks. Those, infected individuals with cirrhosis of the liver were treated for 24 weeks. "From a treatment provider perspective, this is very exciting as we have been waiting for a simple, affordable, robust treatment tolerated by all patients groups, including those whose treatment outcomes are currently poorer, like patients under antiretroviral therapy," explains Pierre Mendiharat, Deputy Operations Director for Médecins Sans Frontières / Doctors Without Borders (MSF). "This will be crucial to expand treatment to the most vulnerable categories of patients in developing countries.”
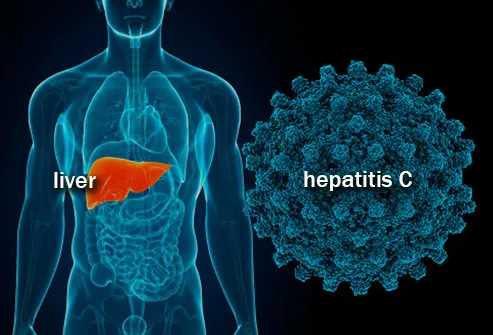
Hepatitis C is an infection that travels through contaminated blood that results in the inflammation of the liver, which occasionally leads to severe liver damage. Over 71 million people have hepatitis C worldwide, causing the deaths of 400,000 per year. Although many treatment s are highly effective and have been present for a number of years, less than three million individuals are on treatment, with more people infected every year than the number being placed on treatment.
Ravidasvir is an oral NS5A inhibitor that is licensed to DNDi by Presidio Pharmaceuticals. Most of the clinical trial participants had HCV genotype 1 (42% of participants) or HCV genotype 3 (53% participants). This confirmed drug combination's effectiveness for those two HCV genotypes. Further trials will test the safety and efficacy of the drug combination in patients infected with the other HCV genotypes.
"Pharco is proud to enable a public health approach to hepatitis C treatment by providing affordable treatments. We look forward to future collaboration in additional clinical trials to confirm the safety and efficacy of ravidasvir," says Dr. Sherine Helmy, CEO, Pharco.
Sources: Science Daily