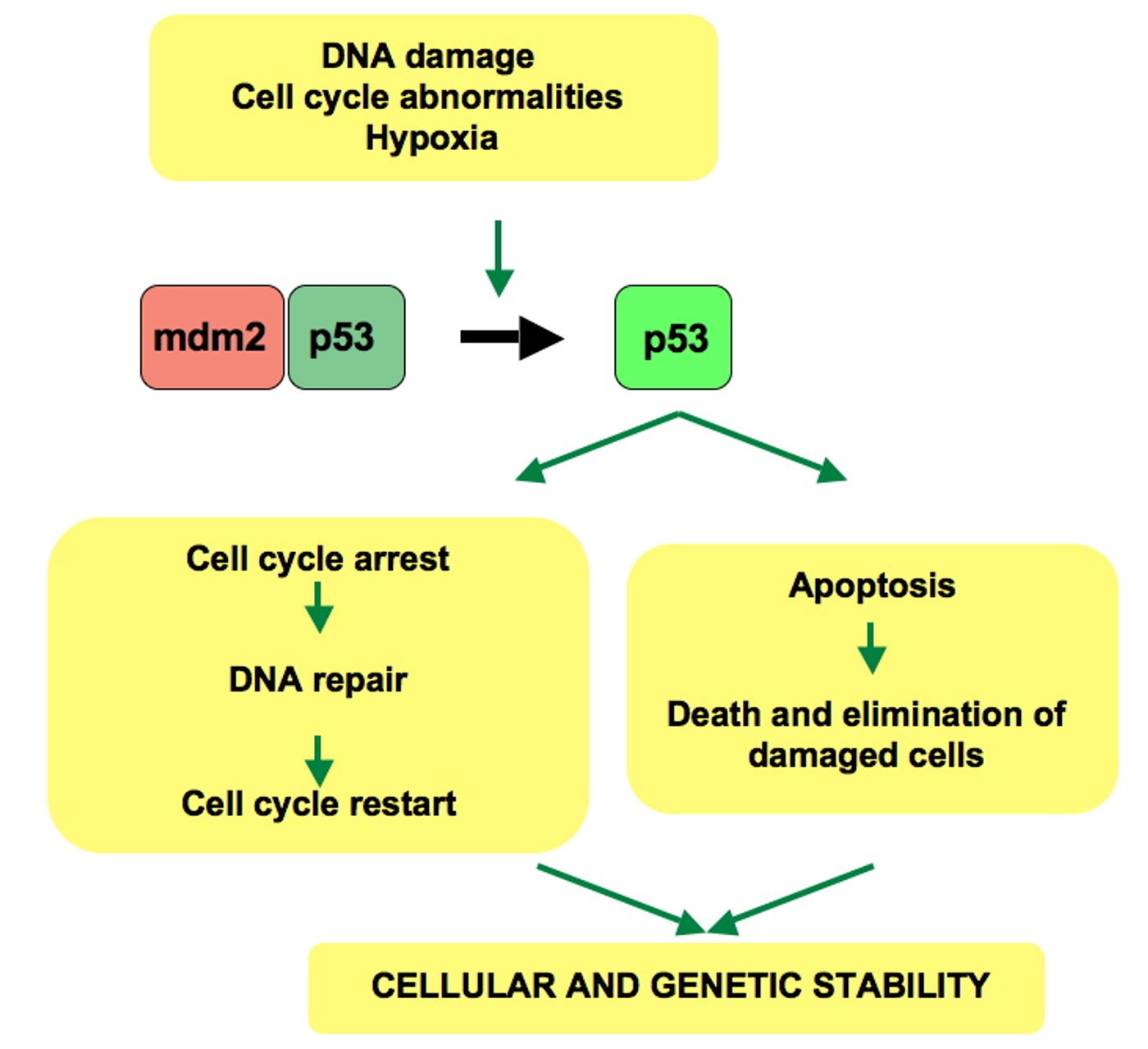
P53 is one of the most well-known and researched tumor suppressor genes in the human genome. It is common knowledge that p53 recognizes and responds to DNA damage in the cell by increasing expression of DNA repair and cell division pathways. If p53 is mutated, it is not able to regulate DNA damage in a cell, leaving the cell prone to potential cancerous mutations. In fact, p53 is one of the most common causes of many different cancer types.
The p53 pathway is an important regulatory decision maker in cells, helping activate DNA repair mechanisms so cells don’t self-destruct due to minor damage. DNA damage is common in DNA replication. With approximately 3 billion nucleotides to replicate, there are bound to be errors. External influences can also cause damage, such as UV light, radiation, or chemicals. Most damage can be repaired quickly without consequence. Other damage can be so severe the cell initiates its own demise. In this way, the p53 pathway is critical to a cell’s survival.
Most research done on the p53 pathway focuses on the genes and proteins involved in regulation and pathway progression. New research from a Stanford team led by Professor of Dermatology Howard Chang, MD, PhD has revealed a new member of the pathway, a novel regulatory long noncoding RNA called DINO. Long noncoding RNAs, or lncRNAs, have been discovered to play critical roles in more and more regulatory pathways in a cell.
Chang and colleagues found that DINO binds to and stabilizes p53 via a positive feedback loop. P53 activates and increases expression of DINO, which then binds to the p53 molecule to stabilize it, allowing it to amplify its signal. P53 is most active in the nucleus where DNA is replicated. DINO is made in the nucleus as well, providing a quicker and more precise response to DNA damage than could be seen from regulatory proteins produced in the cytoplasm.
“DINO expression allows the cell to fine-tune its response to DNA damage and respond appropriately,” said Chang.
The team experimented with increased and decreased expression of DINO. They found that when DINO expression is increased, cells respond as if there was DNA damage to repair. When DINO expression is decreased, cell response to p53 activation is dulled. This places DINO directly within the p53 pathway as a regulatory mechanism of damage response in cells. Implicating RNA in pathways has helped scientists increase their understanding of cell responses and provides a broader range of potential therapies to disease.
“Positive feedback loops are used in many applications, including engineering, to increase the sensitivity of systems and apply thresholds for action,” said Chang. “We believe DINO may play role in cancer development and possibly premature aging by modulating how a cell responds to DNA damage.”
Sources:
Stanford News,
lncRNABlog,
Nature Genetics