Immune cells trap bacteria with DNA, protein
Our innate immune system has a lot of ways to deal with invading pathogens - macrophages gobble up bacteria, for one. Even wilder than that, some immune cells make so-called extracellular traps.
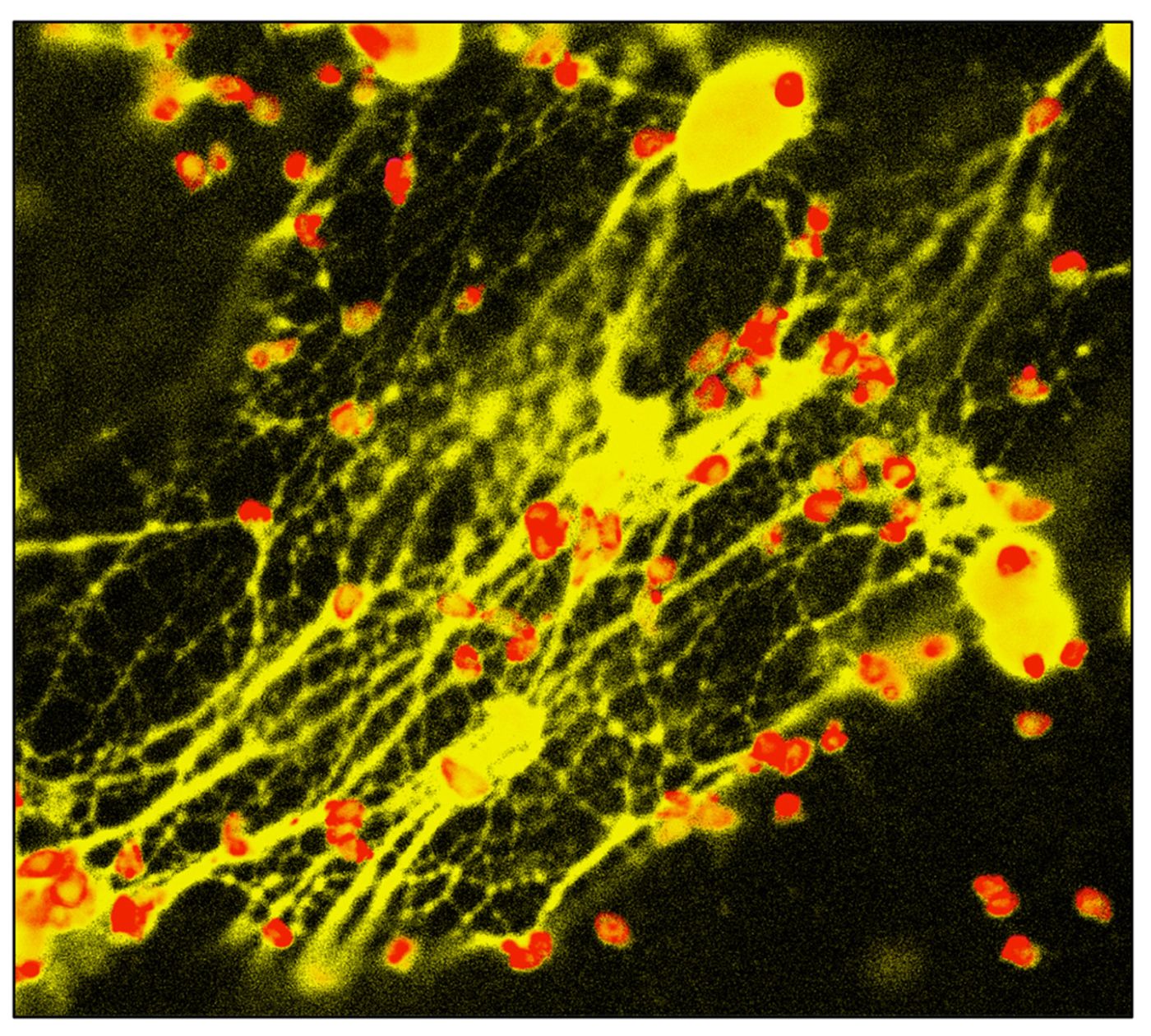
Extracellular traps, ETs for short, were first described as the products of neutrophils - where they were appropriately called NETs, neutrophil extracellular traps. More recently, researchers found that other immune cells also make extracellular traps - now renamed ETs (not the same as the 80’s movie). There’s even a process called ETosis - the cell-death pathway that results in ET formation.
Image: Frontiers in Immunology
But let’s start at the beginning - a very good place to start. NETs were first described in 2004. Like macrophages, neutrophils can also engulf and kill bacteria. Alternatively, they can expel NETs made up of antimicrobial peptides and chromatin (DNA and histone proteins). Researchers know that NETs contain DNA and protein because the NETs can’t kill bacteria if treated with DNase or a protease, respectively. These NETs are active against both Gram-positive and negative bacteria, as well as fungi. Because bacteria are typically negatively charged, it’s possible that the cationic antimicrobial peptides in NETs attract the negatively charged bacteria.
The histones associated with DNA appear to be a particularly important component of NETs. In fact, histones themselves are antimicrobial. Relatively low concentrations of histone H2A kill S. aureus, S. typhimurium, and S. flexneri in just 30 minutes.
ETs, specifically NETs, don’t just kill bacteria, they also inactivate a pathogen’s virulence factors. For example, the proteases in NETs can degrade S. aureus alpha toxin. Likewise, immunofluorescence studies indicate that S. flexneri IpaB and IcsA are degraded by neutrophil elastase, a component of NETs. Interestingly, neutrophil elastase does not degrade OmpA, a bacterial outer membrane protein that is not a virulence factor. This suggests that NETs evolved to target pathogens and their virulence factors, potentially leaving host proteins undamaged.
As with all research, the question is whether or not ETs can be observed in vivo. Or, are they just some weird experimental artifact? One group studied samples from rabbits with experimental shigellosis and humans with spontaneous appendicitis. Sure enough, they found fibrous NET-like structures, and these stained positive for histones, DNA, and neutrophil elastase - all standard components of NETs. What’s more, in the rabbit samples, Shigella localized to the NETs. More recently, NETs have been associated with murine necrotizing fasciitis, murine pneumococcal pneumonia, and bovine mastitis.
Like anything else, too much of a good thing can be bad. While NETs do appear to help clear pathogens from the body, they’re made of DNA - DNA is a danger signal to the body and can trigger inflammation. There are correlations between the presence of NET-associated DNA and conditions such as preeclampsia and lupus. The connection to lupus is particularly interesting because one hallmark of the disease is the presence of autoantigens to self chromatin.
Because neutrophils die soon after entering circulation, and the production of NETs invariably kills the cell, it’s possible that ET formation is actually a form of programmed cell death - right alongside apoptosis and necrosis. Indeed, ETosis (or etosis) begins when reactive oxygen species are formed by NOX2, setting off a program of cell death that ends with ET formation.
Finally, ETs have been in the news this week with the discovery that they’re also made by human blood monocytes. Etosis has already been observed in eosinophils, basophils, mast cells, macrophages, and plasmacytoid dendritic cells, but not in human monocytes. As with neutrophils, etosis is associated with the production of reactive oxygen species and culminates in cell death.
Sources: Journal of Leukocyte Biology, Science, 2004, Science, 2008, Science Daily