Discovery that Can Make Diesel More Eco-friendly
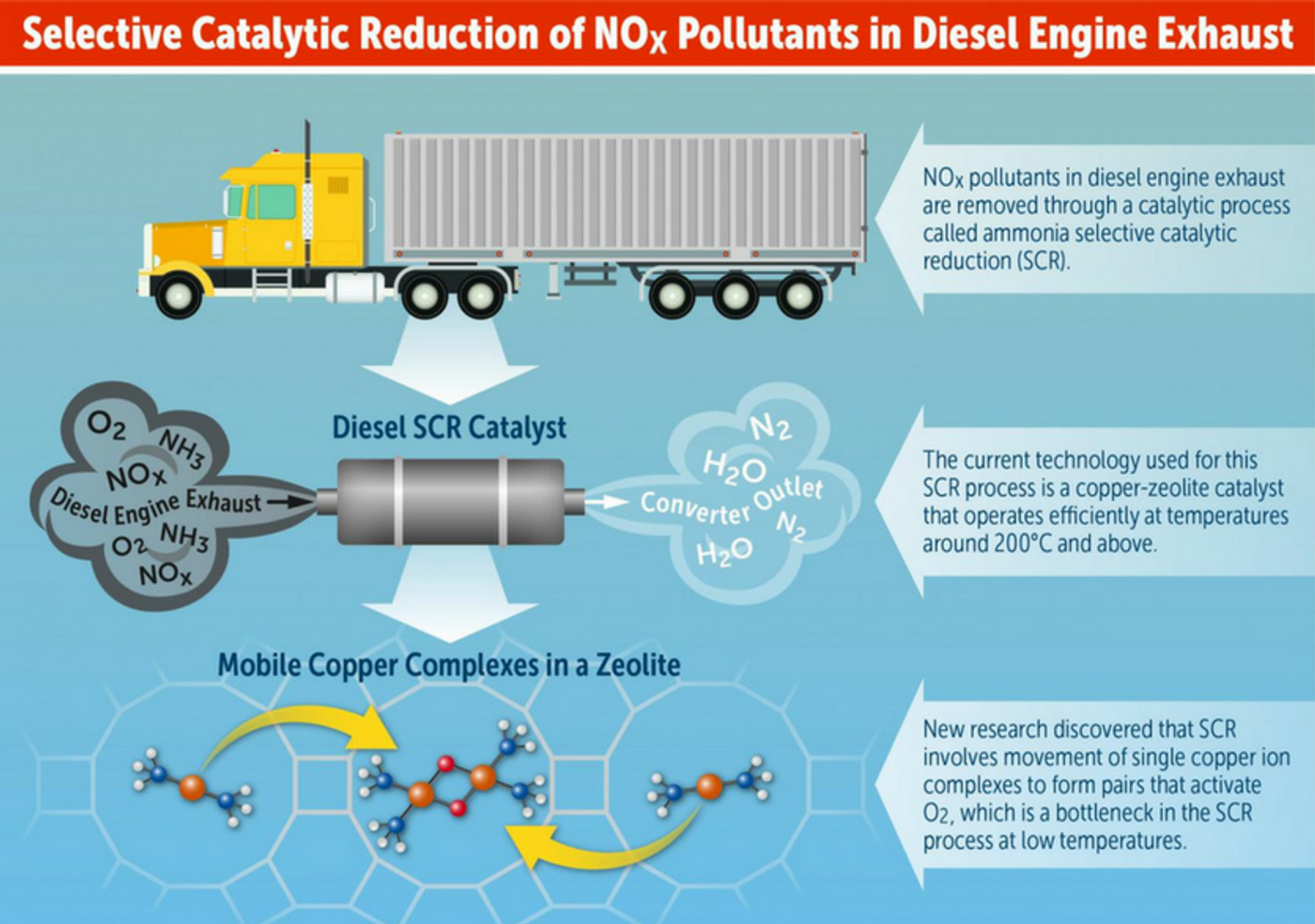
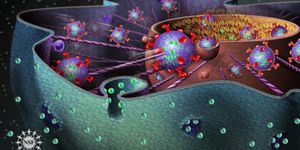
The reaction mechanism that is responsible for NOx catalytic reduction in diesel engine emission-control process. Credit: Purdue University /Maureen Lifton
A team of researchers from Purdue University announced that they have found a way to make diesel engines more eco-friendly. According to their recent publication in Science, they developed a new reaction mechanism that can improve catalyst designs for pollution-control systems for diesel exhaust and therefore reduce emissions of smog-causing nitrogen oxides.
Diesel powered vehicles are popular in Europe, and were relatively well received in North America until Volkswagen’s “diesel gate”. But why diesel? A diesel engine generates more torque than a gasoline engine of the same size, meaning the diesel burner can consume 20% to 40% less fuel when doing the same amount of work. In some European countries such as France, a longstanding subsidy also makes diesel about 15% cheaper than gasoline, making the diesel engine vehicle much more competitive than its gasoline siblings.
But the emissions are what plagues diesel engines. Compared to gasoline vehicles, they produce a higher concentration of nitrogen oxides (NOx), which react with hydrocarbons from the exhaust and forms smog under sunlight. Smog is a mixture of pollutants with ozone (O3) as the main component. While ozone in the upper atmosphere is beneficial because it blocks ultraviolet rays, ground-level ozone has been linked to pre-mature mortality and a range of diseases such as asthma. Therefore, NOx removal remains a challenge for automobile emission reduction.
NOx storage and reduction (NSR) catalysts and selective catalytic reduction (SCR) have been developed over the past 15 years as two promising technologies to overcome this problem. But the drawback in the current SCR design is that it requires relatively high temperatures (200 degrees Celsius and above). During a cold start (meaning starting the engine when it is colder than its normal operating temperature, which is most often the case), NOx reduction is not effective due to a deficiency in the catalysis process. Zeolites, microporous, aluminosilicate minerals, are the main catalytic material used in emission-control systems for diesel engines. Mobile copper complexes in zeolites are responsible for the catalytic reduction of NOx. Copper’s volumetric density under low temperatures does not support the reduction reaction at single sites, leading to the cold start emission problem.
With techniques such as steady-state and transient kinetic measurements, x-ray absorption spectroscopy, and first-principles calculations, Purdue’s chemical engineering team discovered that mobilized copper ions could travel through zeolite windows and form transient ion pairs that participate in a crucial step of catalytic reduction. The tethering of copper to a zeolite framework with aluminum cores reduces the opportunity for each copper ion to participate in the catalytic process. The understanding of this previously unknown mechanism can allow engineers design new catalysts that perform better not only during favorable conditions for the conventional catalyst but also during sustained lower exhaust temperatures.
Commenting on the importance of this discovery, William Schneider Engineering Professor at the University of Notre Dame said "The results here point to a previously unrecognized catalytic mechanism and also point to new directions for discovering better catalysts. This is a reaction of major environmental importance used to clean up the exhaust."
Improved Catalyst Design Possible from New Discovery. Credit: Purdue Engineering