Antibiotics cure infections, right? Well, not always. A new study published in Cell Host & Microbe details how antibiotics can select for the growth of pathogenic bacteria in the gut.
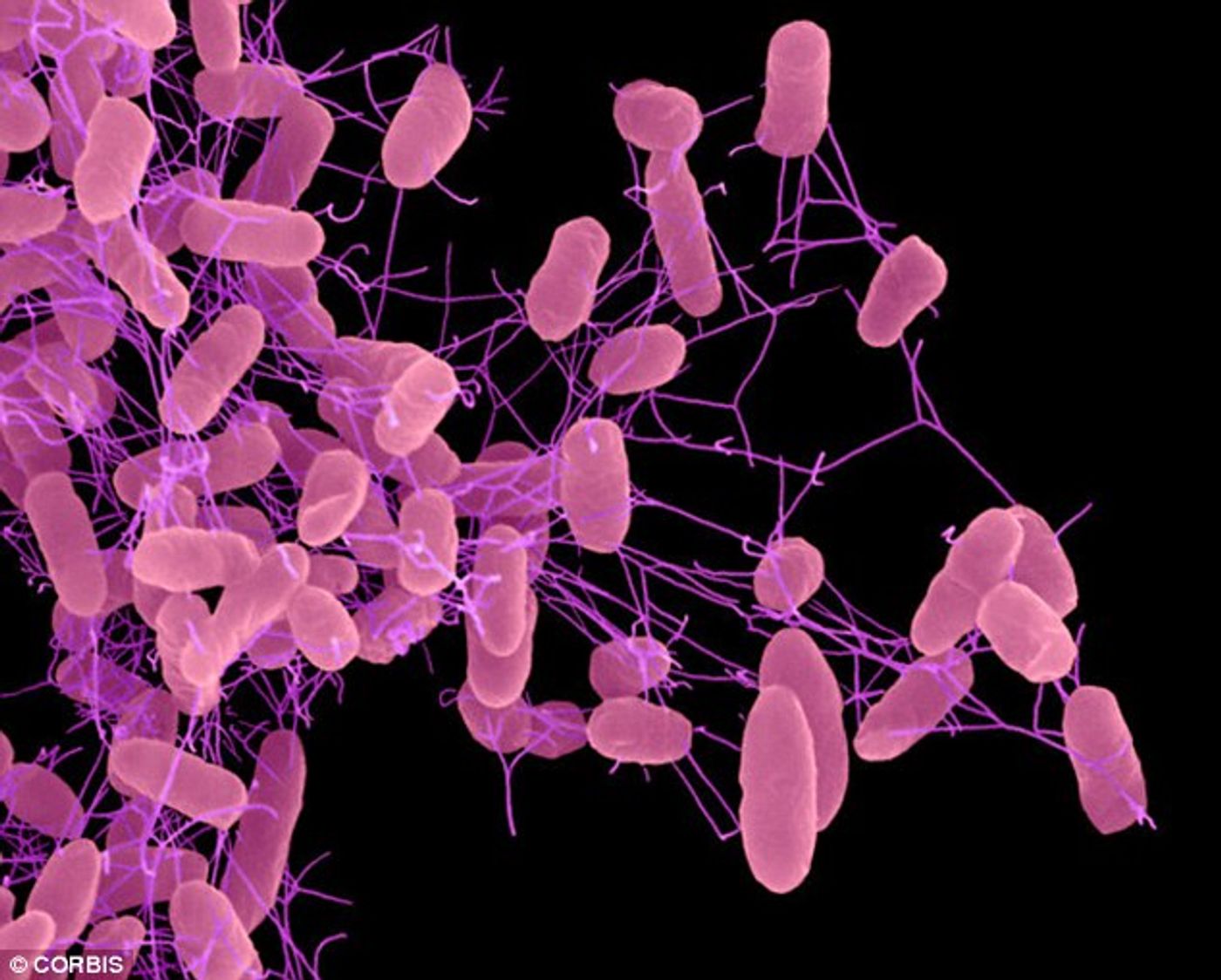
Researchers from UC Davis found that antibiotics create a niche within the gut that helps pathogens like Salmonella flourish. Essentially, antibiotics wipe out the “good” bacteria living in the gut. These bacteria help us break down complex carbohydrates in dietary fiber, producing butyrate. Butyrate is taken up by intestinal epithelial cells and used to convert oxygen to carbon dioxide. Without the butyrate-producing bacteria, oxygen escapes into the intestinal lumen, creating an ideal environment for pathogenic Salmonella to grow.
According to study author Andreas Baumler, “Unlike Clostridia and other beneficial microbes in the gut, which grow anaerobically, or in the complete absence of oxygen, Salmonella flourished in the newly created oxygen-rich microenvironment after antibiotic treatment … in essence, antibiotics enabled pathogens in the gut to breathe”.
The group showed that Salmonella coupled aerobic and nitrate respiration to grow in the guts of streptomycin-treated mice. They constructed a mutant that lacked the ability to use oxygen and nitrate and found that its fitness was severely compromised - after 4 days of infection, nearly 2,000-fold more wild type Salmonella were recovered compared to the mutant. Interestingly, similar numbers of the wild type and mutant cells were recovered from mice that had not been treated with streptomycin. This suggests that the ability to use oxygen and nitrate for respiration contributed to the so-called “post-antibiotic pathogen expansion”.
Interestingly, a decrease in the number of butyrate-producing bacteria has been linked to inflammatory bowel disease. Butyrate was found to decrease the production of proinflammatory cytokines in the gut by inhibiting the activity of NF-κB. Similarly, one
study found that butyrate increased the number of T regulatory cells in the gut, possibly alleviating inflammation. According to study author Hiroshi Ohno, “butyrate is natural and safe as a therapy and in addition to that it is cheap, which could reduce costs for both patients and society”.
Sources: UC Davis,
Cell Host & Microbe,
Gut,
Science 2.0