FDA approves a new drug for an advanced type of skin cancer
As cancer is one of the most challenging diseases in our world, many efforts have been put into researching new ways for treatment to find new drugs that could help more patients worldwide. Anticancer immunotherapy drugs are one class of drugs that have been developed recently and that have successfully helped in treating different types of cancers, no wonder that the Nobel prize in medicine/physiology this year has been awarded to the two doctors who contributed in discovering this class.
Recently the U.S. Food and Drug Administration has approved a new drug called Libtayo (cemiplimab-rwlc) which is a member of the anticancer immunotherapy class of drugs, it is an injection for intravenous use for the treatment of patients with metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC who cannot be treated with surgery or radiation.
This drug is the first and only drug to treat this type of skin cancer (CSCC) which is the second most common type of cancer in the United States and represents 20% of all non-melanoma skin cancer. It is usually developed by regular exposure to the sun or ultraviolet radiation. It can cause disfigurement and other local complications as bleeding or infection. Also, it can metastasize to other organs in the body which can make it life-threatening.
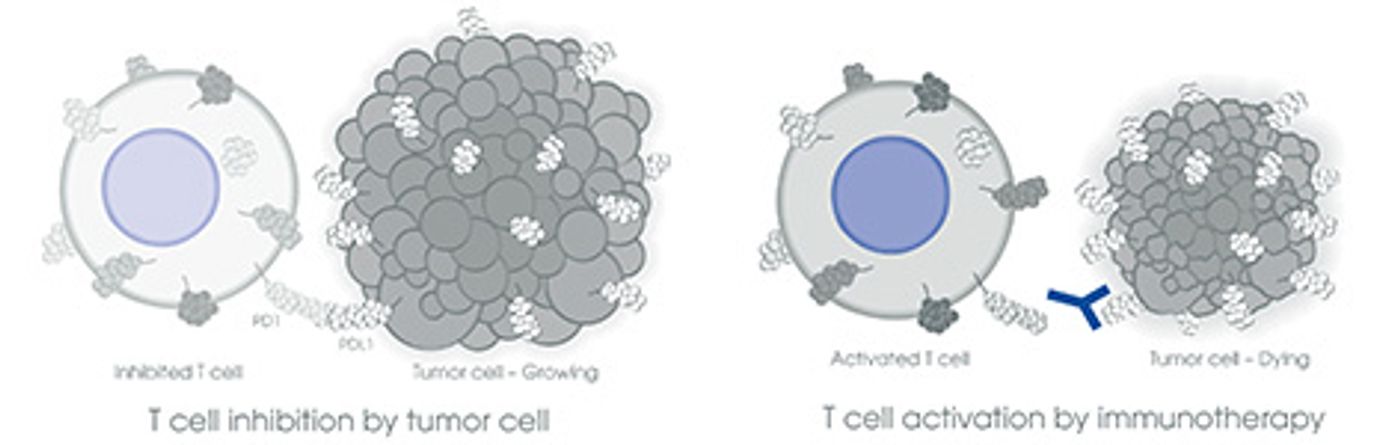
Libtayo works by blocking the PD-1 / PD-L1 pathway, by doing this it can help the immune system fights cancer.
The PD-1 is a protein receptor expressed on T-cells, it binds to its ligand PD-L1 which is commonly expressed on macrophages and dendritic cells. They both belong to the family of checkpoints that function to ensure that the immune system only reacts at the right time.
Cancer cells express a PD-L1 receptor that after binding to PD-1, this inhibits T cells making them unable to destroy the cancer cells and they keep on growing.
Two open-label clinical trials were done to study the efficacy and safety of Libtayo with 108 patients included (75 with metastatic disease and 33 with the locally-advanced disease). The results showed that the tumors shrunk or disappeared in 47.2% of all patients treated.
Libtayo side effects include fatigue, rash, and diarrhea. It can also cause severe or life-threatening side effects due to its effect on normal tissues and organs that can impair their functions, like lung problems (pneumonitis), intestinal problems (colitis), liver problems (hepatitis), hormone gland problems (endocrinopathies), skin (dermatologic) problems and kidney problems. Patients should also be monitored for infusion-related reactions.
It is not safe in pregnancy as it can cause harm to the fetus.
“We are continuing to see a shift in oncology toward identifying and developing drugs aimed at a specific molecular target. With the Libtayo approval, the FDA has approved six immune checkpoint inhibitors targeting the PD-1 / PD-L1 pathway for treating a variety of tumors, from bladder to head and neck cancer, and now advanced CSCC,” said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “This type of cancer can be difficult to treat effectively when it is advanced, and it is important that we continue to bring new treatment options to patients.”
Watch the video below to learn more about cancer immunotherapy.